Prevent damages from Injection Attacks with Software Correction Technology
Thu, Nov 14, 2024-
Tags
Transforming Anion Exchange Membranes in Water Electrolysis for Green Hydrogen Production
A new polymer-based anion exchange membrane improves performance and durability, which is essential for producing green hydrogen
A group of researchers has implemented polyphenylene-based anion exchange membranes (AEMs) poised to make hydrogen production more efficient and durable. Its robust hydrophobic design enables effective ion transport while resisting chemical degradation. This supports its potential for durable, high-efficiency use in AEM water electrolyzers, making it a promising component in sustainable hydrogen production applications, which would advance the goal of a carbon-free energy future.
Hydrogen is a promising energy source due to its high energy density and zero carbon emissions, making it a key element in the shift toward carbon neutrality. Traditional hydrogen production methods, like coal gasification and steam methane reforming, release carbon dioxide, undermining environmental goals. Electrochemical water splitting, which yields only hydrogen and oxygen, presents a cleaner alternative. While proton exchange membrane (PEM) and alkaline water electrolyzers (AWEs) are available, they face limitations in either cost or efficiency. PEM electrolyzers, for instance, rely on costly platinum group metals (PGMs) as catalysts, whereas AWEs often operate at lower current densities and efficiencies.
Anion exchange membrane water electrolyzers (AEMWEs) combine benefits of both PEM and AWEs, using low-cost, non-PGM catalysts while supporting higher current densities and energy conversion efficiencies. However, AEMs face technical challenges, especially degradation under alkaline conditions, which impacts long-term stability. Advances in AEM materials, particularly those enhancing chemical durability, conductivity, and mechanical strength, are critical to overcoming these challenges.
To address these issues, Professor Kenji Miyatake from Waseda University, Japan working alongside researchers at the University of Yamanashi, developed a new anion exchange membrane (AEM) with durable hydrophobic components. They published their study in the journal Advanced Energy Materials on 29 September 2024. High hydroxide ion (OH ̅) conductivity, which is essential for excellent performance in AEM water electrolyzers (AEMWEs), is another feature of this membrane, which is made to withstand extreme alkaline conditions. Miyatake stated, “The polymer-based membrane used in this study satisfies the fundamental requirement for robust, effective materials in the production of green hydrogen to be used in water electrolysis.”
The incorporation of 3,3”-dichloro-2′,5′-bis(trifluoromethyl)-1,1′:4′,1”-terphenyl (TFP) monomers into the polyphenylene backbone of the membrane is a crucial aspect of this breakthrough. Since its composition enhances stability, it possesses the capacity to endure more than 810 hours of exposure to high concentrations of potassium hydroxide at 80 °C which shows its durability in industrial applications.
The membrane demonstrated consistent performance during water electrolyzer testing, sustaining a constant current density of 1.0 A.cm–² for over 1,000 hours with minimal voltage change. According to Miyatake, “The durability shown here is an encouraging sign that our membrane can help reduce costs in hydrogen production.”
Further, the membrane’s OH ̅ conductivity reached 168.7 mS.cm-1 at 80 °C, surpassing the values mentioned in earlier research studies. This high conductivity is critical for achieving high current densities needed to make hydrogen production efficient. By combining durability with such high conductivity, the team believes this material design marks an important advance toward scalable and affordable hydrogen production.
With a tensile strength of 27.4 MPa and an elongation capacity of 125.6%, the membranes offer strong resilience, beneficial for stable performance over time. The durability and efficiency of these AEMs make them a valuable component in sustainable hydrogen production, supporting carbon-neutral energy initiatives. These results hold promise for applications involving green hydrogen.
The study successfully demonstrates that polyphenylene-based AEMs with hydrophobic components significantly enhance stability and exhibit high hydroxide ion conductivity with superior alkaline stability, minimizing degradation even in challenging environments. The membrane enables stable performance over prolonged operation at high current densities, marking it as an efficient, cost-effective option for green hydrogen production in AEM water electrolyzers.
This research has brought us one step closer to a future of sustainable energy.
Reference
Authors: Fanghua Liu1, Kenji Miyatake1,2,3, Ahmed Mohamed Ahmed Mahmoud1, Vikrant Yadav1, Fang Xian1, Lin Guo1, Chun Yik Wong1, Toshio Iwataki2, Yuto Shirase2, Katsuyoshi Kakinuma2, and Makoto Uchida2
Title of original paper: Polyphenylene-Based Anion Exchange Membranes with Robust Hydrophobic Components Designed for High-Performance and Durable Anion Exchange Membrane Water Electrolyzers Using Non-PGM Anode Catalysts
Journal: Advanced Energy Materials
DOI: https://doi.org/10.1002/aenm.202404089
Affiliations
1Clean Energy Research Center, University of Yamanashi, Japan
2Hydrogen and Fuel Cell Nanomaterials Center, University of Yamanashi, Japan
3Department of Applied Chemistry, Waseda University, Japan
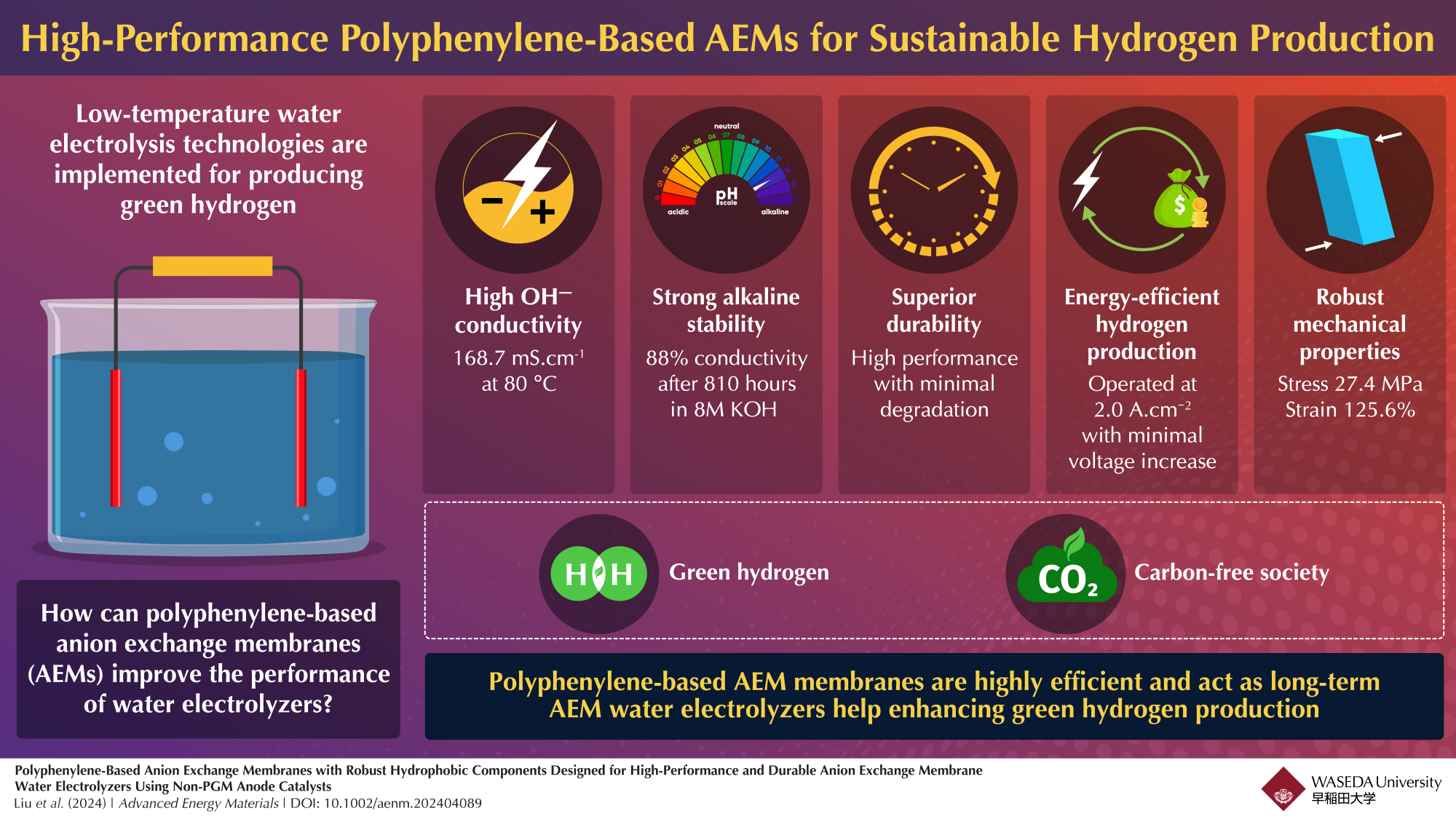
Image title: High-Performance Polyphenylene-Based AEMs for Sustainable Hydrogen Production
Image caption: Polyphenylene-based AEMs showed enhanced durability and conductivity in water electrolyzers improving green hydrogen production
Image credit: Kenji Miyatake from Waseda University, Japan
License type: Original content
Usage restrictions: Cannot be reused without permission
About Professor Kenji Miyatake from Waseda University, Japan
Dr. Kenji Miyatake is a leading scientist at Waseda University and the University of Yamanashi in Japan, where he specializes in materials science and ion-conductive polymers for energy applications. His work focuses on innovative membrane design to advance fuel cell and hydrogen production technologies, which are key in the global shift towards carbon-free energy. He also works on all-solid-state air batteries using ion-conductive polymers and redox active organic molecules.













