Abstract
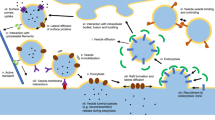
Neurotransmitters in the terminal bouton of a presynaptic neuron are stored in vesicles, which diffuse in the cytoplasm and, after a stimulation signal is received, fuse with the membrane and release its contents into the synaptic cleft. It is commonly assumed that vesicles belong to three pools whose content is gradually exploited during the stimulation. This article presents a model that relies on the assumption that the release ability is associated with the vesicle location in the bouton. As a modeling tool, partial differential equations are chosen as they allow one to express the continuous dependence of the unknown vesicle concentration on both the time and space variables. The model represents the synthesis, concentration-gradient-driven diffusion, and accumulation of vesicles as well as the release of neuroactive substances into the cleft. An initial and boundary value problem is numerically solved using the finite element method (FEM) and the simulation results are presented and discussed. Simulations were run for various assumptions concerning the parameters that govern the synthesis and diffusion processes. The obtained results are shown to be consistent with those obtained for a compartment model based on ordinary differential equations. Such studies can be helpful in gaining a deeper understanding of synaptic processes including physiological pathologies. Furthermore, such mathematical models can be useful for estimating the biological parameters that are included in a model and are hard or impossible to measure directly.
Similar content being viewed by others
References
Aristizabal F, Glavinovic MI (2004) Simulation and parameter estimation of dynamics of synaptic depression. Biol Cybern 90: 3–18
Bielecki A, Kalita P (2008) Model of neurotransmitter fast transport in axon terminal of presynaptic neuron. J Math Biol 56: 559–576
Chang S, Popov SV (1999) Longrange signaling within growing neurites mediated by neurotrophin-3. Neurobiology 96: 4095–4100
Ciarlet PG (2002) The finite element method for elliptic problems. SIAM, Philadelphia
Edwards RH (1992) The transport of neurotransmitters into synaptic vesicles. Curr Opin Neurobiol 2: 586–594
Fall CP, Marland ES, Wagner JM, Tyson JJ (2002) Computational cell biology. Springer, Berlin, Heidelberg, New York
Heuser JE (1977) Synaptic vesicle exocytosis revealed in quick-frozen frog neuromuscular junctions treated with 4-aminopyridine and given a single electrical shock. In: Cowan WM, Ferrendelli JA (eds) Neuroscience symposia, vol 2. Society for Neuroscience, Bethesda, pp 215–239
Keener J, Sneyd J (1998) Mathematical physiology. Springer, Berlin, Heidelberg, New York
McGuiness TL, Brady ST, Gruner JA, Sugimori M, Llinas R, Greengard P (1989) Phosphorylation-dependent inhibition by synapsin I of organelle movement in squid axoplasm. J Neurosci 9: 4138–4149
Morris SA, Schmid SL (1995) Synaptic vesicle recycling: the Ferrari of endocytosis?. Curr Biol 5: 113–115
Murthy VN, Stevens CF (1998) Synaptic vesicles retain their identity through the endocytic cycle. Nature 392: 497–501
Oheim M, Loerke D, Stuhmer W, Chow RH (1999) Multiple stimulation-dependent processes regulate the size of the releasable pool of vesicles. Eur Biophys J 28: 91–101
Okuda T, Haga T, Kanai Y, Endou H, Ishihara T, Katsura I (2000) Identification and characterization of the high affinity choline transporter. Nat Neurosci 3: 120–125
Parsons TD, Coorsen JR, Horstmann H, Almers W (1995) Docked granules, the exocytic burst, and the need for ATP hydrolysis in endoctrine cells. Neuron 15: 1085–1096
Rizo J, Rosenmund C (2008) Synaptic vesicle fusion. Nat Struct Mol Biol 15: 665–674
Rizzoli SO, Betz WJ (2005) Synaptic vesicle pools. Nat Rev Neurosci 6: 57–69
Salin-Pascual RJ, Jimenez-Anguiano A (1995) Vesamicol, an acetylcholine uptake blocker in presynaptic vesicles, suppresses rapid eye movement (REM) sleep in the rat. Psychopharmacology 121: 485–487
Squire LR, Bloom FE, Spitzer NC, duLac S, Ghosh A, Berg D (2008) Fundamental neuroscience. Academic Press, New York
Steyer JA, Horstmann H, Almers W (1997) Transport, docking and exocytosis of single granules in live chromaffin cells. Nature 388: 474–478
Sudhof TC (2004) The synaptic vesicle cycle. Annu Rev Neurosci 27: 509–547
Triangle library: http://www.cs.cmu.edu/~quake/triangle.html. Last accessed 11 March 2010
Vitale ML, Seward EP, Trifaró JM (1995) Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron 14: 353–363
von Gersdorff H, Matthews G (1994) Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature 370: 652–655
Whittaker VP (1988) Model cholinergic systems: an overview. In: Whittaker VP (eds) Series: Handbook of experimental pharmacology, vol 86. Springer, Berlin, pp 3–22
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bielecki, A., Kalita, P., Lewandowski, M. et al. Numerical simulation for a neurotransmitter transport model in the axon terminal of a presynaptic neuron. Biol Cybern 102, 489–502 (2010). https://doi.org/10.1007/s00422-010-0380-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-010-0380-z