Predicting and Manipulating Cone Responses to Naturalistic Inputs
- PMID: 34949692
- PMCID: PMC8883858
- DOI: 10.1523/JNEUROSCI.0793-21.2021
Predicting and Manipulating Cone Responses to Naturalistic Inputs
Abstract
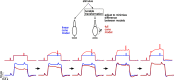
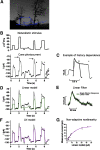
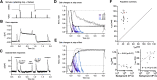
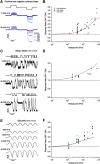
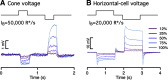
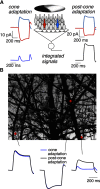
Primates explore their visual environment by making frequent saccades, discrete and ballistic eye movements that direct the fovea to specific regions of interest. Saccades produce large and rapid changes in input. The magnitude of these changes and the limited signaling range of visual neurons mean that effective encoding requires rapid adaptation. Here, we explore how macaque cone photoreceptors maintain sensitivity under these conditions. Adaptation makes cone responses to naturalistic stimuli highly nonlinear and dependent on stimulus history. Such responses cannot be explained by linear or linear-nonlinear models but are well explained by a biophysical model of phototransduction based on well-established biochemical interactions. The resulting model can predict cone responses to a broad range of stimuli and enables the design of stimuli that elicit specific (e.g., linear) cone photocurrents. These advances will provide a foundation for investigating the contributions of cone phototransduction and post-transduction processing to visual function.SIGNIFICANCE STATEMENT We know a great deal about adaptational mechanisms that adjust sensitivity to slow changes in visual inputs such as the rising or setting sun. We know much less about the rapid adaptational mechanisms that are essential for maintaining sensitivity as gaze shifts around a single visual scene. We characterize how phototransduction in cone photoreceptors adapts to rapid changes in input similar to those encountered during natural vision. We incorporate these measurements into a quantitative model that can predict cone responses across a broad range of stimuli. This model not only shows how cone phototransduction aids the encoding of natural inputs but also provides a tool to identify the role of the cone responses in shaping those of downstream visual neurons.
Keywords: neural coding; photoreceptors; phototransduction; retina; sensory processing.
Copyright © 2022 the authors.
Figures






Similar articles
-
Predictably manipulating photoreceptor light responses to reveal their role in downstream visual responses.Elife. 2024 Nov 5;13:RP93795. doi: 10.7554/eLife.93795. Elife. 2024. PMID: 39498955 Free PMC article.
-
Probing Computation in the Primate Visual System at Single-Cone Resolution.Annu Rev Neurosci. 2019 Jul 8;42:169-186. doi: 10.1146/annurev-neuro-070918-050233. Epub 2019 Mar 11. Annu Rev Neurosci. 2019. PMID: 30857477 Free PMC article. Review.
-
Macaque V1 activity during natural vision: effects of natural scenes and saccades.J Neurophysiol. 2008 Feb;99(2):460-72. doi: 10.1152/jn.00612.2007. Epub 2007 Dec 12. J Neurophysiol. 2008. PMID: 18077668 Free PMC article.
-
Sensory sluggishness dissociates saccadic, manual, and perceptual responses: an S-cone study.J Vis. 2008 Jun 24;8(8):10.1-13. doi: 10.1167/8.8.10. J Vis. 2008. PMID: 18831633
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
Cited by
-
Predictably manipulating photoreceptor light responses to reveal their role in downstream visual responses.Elife. 2024 Nov 5;13:RP93795. doi: 10.7554/eLife.93795. Elife. 2024. PMID: 39498955 Free PMC article.
-
Biophysical neural adaptation mechanisms enable artificial neural networks to capture dynamic retinal computation.Nat Commun. 2024 Jul 16;15(1):5957. doi: 10.1038/s41467-024-50114-5. Nat Commun. 2024. PMID: 39009568 Free PMC article.
-
Adaptation in cone photoreceptors contributes to an unexpected insensitivity of primate On parasol retinal ganglion cells to spatial structure in natural images.Elife. 2022 Mar 14;11:e70611. doi: 10.7554/eLife.70611. Elife. 2022. PMID: 35285798 Free PMC article.
-
Applying Super-Resolution and Tomography Concepts to Identify Receptive Field Subunits in the Retina.PLoS Comput Biol. 2024 Sep 3;20(9):e1012370. doi: 10.1371/journal.pcbi.1012370. eCollection 2024 Sep. PLoS Comput Biol. 2024. PMID: 39226328 Free PMC article.
-
Regional tuning of photoreceptor adaptation in the primate retina.Nat Commun. 2024 Oct 12;15(1):8821. doi: 10.1038/s41467-024-53061-3. Nat Commun. 2024. PMID: 39394185 Free PMC article.
References
-
- Arshavsky VY, Lamb TD, Pugh ENJ (2002) G proteins and phototransduction. Annu Rev Physiol 64:153–187. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
