Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts
- PMID: 32119825
- PMCID: PMC7097845
- DOI: 10.1016/S2214-109X(20)30074-7
Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts
Erratum in
-
Correction to Lancet Glob Health 2020; published online Feb 28. https://doi.org/10.1016/S2214-109X(20)30074-7.Lancet Glob Health. 2021 May;9(5):e597. doi: 10.1016/S2214-109X(20)30083-8. Epub 2020 Mar 5. Lancet Glob Health. 2021. PMID: 32145764 Free PMC article. No abstract available.
Abstract
Background: Isolation of cases and contact tracing is used to control outbreaks of infectious diseases, and has been used for coronavirus disease 2019 (COVID-19). Whether this strategy will achieve control depends on characteristics of both the pathogen and the response. Here we use a mathematical model to assess if isolation and contact tracing are able to control onwards transmission from imported cases of COVID-19.
Methods: We developed a stochastic transmission model, parameterised to the COVID-19 outbreak. We used the model to quantify the potential effectiveness of contact tracing and isolation of cases at controlling a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-like pathogen. We considered scenarios that varied in the number of initial cases, the basic reproduction number (R0), the delay from symptom onset to isolation, the probability that contacts were traced, the proportion of transmission that occurred before symptom onset, and the proportion of subclinical infections. We assumed isolation prevented all further transmission in the model. Outbreaks were deemed controlled if transmission ended within 12 weeks or before 5000 cases in total. We measured the success of controlling outbreaks using isolation and contact tracing, and quantified the weekly maximum number of cases traced to measure feasibility of public health effort.
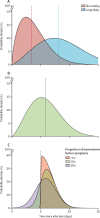
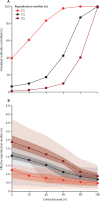
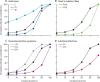
Findings: Simulated outbreaks starting with five initial cases, an R0 of 1·5, and 0% transmission before symptom onset could be controlled even with low contact tracing probability; however, the probability of controlling an outbreak decreased with the number of initial cases, when R0 was 2·5 or 3·5 and with more transmission before symptom onset. Across different initial numbers of cases, the majority of scenarios with an R0 of 1·5 were controllable with less than 50% of contacts successfully traced. To control the majority of outbreaks, for R0 of 2·5 more than 70% of contacts had to be traced, and for an R0 of 3·5 more than 90% of contacts had to be traced. The delay between symptom onset and isolation had the largest role in determining whether an outbreak was controllable when R0 was 1·5. For R0 values of 2·5 or 3·5, if there were 40 initial cases, contact tracing and isolation were only potentially feasible when less than 1% of transmission occurred before symptom onset.
Interpretation: In most scenarios, highly effective contact tracing and case isolation is enough to control a new outbreak of COVID-19 within 3 months. The probability of control decreases with long delays from symptom onset to isolation, fewer cases ascertained by contact tracing, and increasing transmission before symptoms. This model can be modified to reflect updated transmission characteristics and more specific definitions of outbreak control to assess the potential success of local response efforts.
Funding: Wellcome Trust, Global Challenges Research Fund, and Health Data Research UK.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
What further should be done to control COVID-19 outbreaks in addition to cases isolation and contact tracing measures?BMC Med. 2020 Mar 13;18(1):80. doi: 10.1186/s12916-020-01551-8. BMC Med. 2020. PMID: 32164708 Free PMC article. No abstract available.
-
Deciphering the power of isolation in controlling COVID-19 outbreaks.Lancet Glob Health. 2020 Apr;8(4):e452-e453. doi: 10.1016/S2214-109X(20)30085-1. Lancet Glob Health. 2020. PMID: 32199105 Free PMC article. No abstract available.
-
Use of antiviral drugs to reduce COVID-19 transmission.Lancet Glob Health. 2020 May;8(5):e639-e640. doi: 10.1016/S2214-109X(20)30114-5. Epub 2020 Mar 19. Lancet Glob Health. 2020. PMID: 32199468 Free PMC article. No abstract available.
-
Feasibility of controlling COVID-19.Lancet Glob Health. 2020 Jun;8(6):e774. doi: 10.1016/S2214-109X(20)30129-7. Epub 2020 Apr 30. Lancet Glob Health. 2020. PMID: 32359416 Free PMC article. No abstract available.
-
On the fallibility of simulation models in informing pandemic responses - Authors' reply.Lancet Glob Health. 2020 Jun;8(6):e778-e779. doi: 10.1016/S2214-109X(20)30217-5. Epub 2020 Apr 30. Lancet Glob Health. 2020. PMID: 32359418 Free PMC article. No abstract available.
-
On the fallibility of simulation models in informing pandemic responses.Lancet Glob Health. 2020 Jun;8(6):e776-e777. doi: 10.1016/S2214-109X(20)30219-9. Epub 2020 Apr 30. Lancet Glob Health. 2020. PMID: 32359419 Free PMC article. No abstract available.
Similar articles
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Ceftazidime with avibactam for treating severe aerobic Gram-negative bacterial infections: technology evaluation to inform a novel subscription-style payment model.Health Technol Assess. 2024 Oct;28(73):1-230. doi: 10.3310/YAPL9347. Health Technol Assess. 2024. PMID: 39487661 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Topical fluoride as a cause of dental fluorosis in children.Cochrane Database Syst Rev. 2024 Jun 20;6(6):CD007693. doi: 10.1002/14651858.CD007693.pub3. Cochrane Database Syst Rev. 2024. PMID: 38899538 Review.
Cited by
-
Applicability of time fractional derivative models for simulating the dynamics and mitigation scenarios of COVID-19.Chaos Solitons Fractals. 2020 Sep;138:109959. doi: 10.1016/j.chaos.2020.109959. Epub 2020 Jun 4. Chaos Solitons Fractals. 2020. PMID: 32834580 Free PMC article.
-
Will an imperfect vaccine curtail the COVID-19 pandemic in the U.S.?Infect Dis Model. 2020;5:510-524. doi: 10.1016/j.idm.2020.07.006. Epub 2020 Aug 6. Infect Dis Model. 2020. PMID: 32835142 Free PMC article.
-
Active testing of groups at increased risk of acquiring SARS-CoV-2 in Canada: costs and human resource needs.CMAJ. 2020 Oct 5;192(40):E1146-E1155. doi: 10.1503/cmaj.201128. Epub 2020 Sep 9. CMAJ. 2020. PMID: 32907820 Free PMC article.
-
Feasibility of large-scale population testing for SARS-CoV-2 detection by self-testing at home.Sci Rep. 2021 May 10;11(1):9819. doi: 10.1038/s41598-021-89236-x. Sci Rep. 2021. PMID: 33972607 Free PMC article.
-
Five approaches to the suppression of SARS-CoV-2 without intensive social distancing.medRxiv [Preprint]. 2020 Aug 1:2020.07.30.20165159. doi: 10.1101/2020.07.30.20165159. medRxiv. 2020. Update in: Proc Biol Sci. 2021 Apr 28;288(1949):20203074. doi: 10.1098/rspb.2020.3074 PMID: 32766603 Free PMC article. Updated. Preprint.
References
-
- WHO . World Health Organization; 2020. Novel coronavirus (2019-nCoV) situation report 16.https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- WHO . World Health Organization; 2020. Novel coronavirus (2019-nCoV) situation report 2.https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- European Centre for Disease Prevention and Control . European Centre for Disease Prevention and Control; 2020. Public health management of persons having had contact with novel coronavirus cases in the European Union.https://www.ecdc.europa.eu/en/publications-data/public-health-management...
-
- Quilty B, Clifford S, CCMID nCoV working group. Flasche S, Eggo RM. Effectiveness of airport screening at detecting travellers infected with 2019-nCoV. 2020. https://cmmid.github.io/ncov/airport_screening_report/airport_screening_... - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

