Therapeutic Cancer Vaccination with Ex Vivo RNA-Transfected Dendritic Cells-An Update
- PMID: 31979205
- PMCID: PMC7076681
- DOI: 10.3390/pharmaceutics12020092
Therapeutic Cancer Vaccination with Ex Vivo RNA-Transfected Dendritic Cells-An Update
Abstract
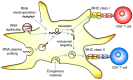
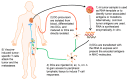
Over the last two decades, dendritic cell (DC) vaccination has been studied extensively as active immunotherapy in cancer treatment and has been proven safe in all clinical trials both with respect to short and long-term side effects. For antigen-loading of dendritic cells (DCs) one method is to introduce mRNA coding for the desired antigens. To target the whole antigenic repertoire of a tumor, even the total tumor mRNA of a macrodissected biopsy sample can be used. To date, reports have been published on a total of 781 patients suffering from different tumor entities and HIV-infection, who have been treated with DCs loaded with mRNA. The majority of those were melanoma patients, followed by HIV-infected patients, but leukemias, brain tumors, prostate cancer, renal cell carcinomas, pancreatic cancers and several others have also been treated. Next to antigen-loading, mRNA-electroporation allows a purposeful manipulation of the DCs' phenotype and function to enhance their immunogenicity. In this review, we intend to give a comprehensive summary of what has been published regarding clinical testing of ex vivo generated mRNA-transfected DCs, with respect to safety and risk/benefit evaluations, choice of tumor antigens and RNA-source, and the design of better DCs for vaccination by transfection of mRNA-encoded functional proteins.
Keywords: RNA; clinical trial; dendritic cells; electroporation; immunotherapy; therapeutic vaccination.
Conflict of interest statement
The authors declare the following potential conflict of interest: G.S., N.S., and J.D. are named as inventors on a patent on caIKK-RNA-electroporated DCs (WO/2012/055551). The funders had no role in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients.Clin Cancer Res. 2009 May 15;15(10):3366-75. doi: 10.1158/1078-0432.CCR-08-2982. Epub 2009 May 5. Clin Cancer Res. 2009. PMID: 19417017
-
Cancer immunotherapy with mRNA-transfected dendritic cells.Immunol Rev. 2004 Jun;199:251-63. doi: 10.1111/j.0105-2896.2004.00139.x. Immunol Rev. 2004. PMID: 15233739 Review.
-
Immunotherapy of cancer with dendritic cells loaded with tumor antigens and activated through mRNA electroporation.Methods Mol Biol. 2010;629:405-52. doi: 10.1007/978-1-60761-657-3_27. Methods Mol Biol. 2010. PMID: 20387165 Clinical Trial.
-
Preparing clinical-grade myeloid dendritic cells by electroporation-mediated transfection of in vitro amplified tumor-derived mRNA and safety testing in stage IV malignant melanoma.J Transl Med. 2006 Aug 15;4:35. doi: 10.1186/1479-5876-4-35. J Transl Med. 2006. PMID: 16911798 Free PMC article.
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
Cited by
-
A synthetic DNA template for fast manufacturing of versatile single epitope mRNA.Mol Ther Nucleic Acids. 2022 Aug 17;29:943-954. doi: 10.1016/j.omtn.2022.08.021. eCollection 2022 Sep 13. Mol Ther Nucleic Acids. 2022. PMID: 36159589 Free PMC article.
-
A One-Armed Phase I Dose Escalation Trial Design: Personalized Vaccination with IKKβ-Matured, RNA-Loaded Dendritic Cells for Metastatic Uveal Melanoma.Front Immunol. 2022 Feb 4;13:785231. doi: 10.3389/fimmu.2022.785231. eCollection 2022. Front Immunol. 2022. PMID: 35185883 Free PMC article.
-
mRNA vaccines for cancer immunotherapy.Front Immunol. 2022 Dec 14;13:1029069. doi: 10.3389/fimmu.2022.1029069. eCollection 2022. Front Immunol. 2022. PMID: 36591226 Free PMC article. Review.
-
RNA in Cancer Immunotherapy: Unlocking the Potential of the Immune System.Clin Cancer Res. 2022 Sep 15;28(18):3929-3939. doi: 10.1158/1078-0432.CCR-21-3304. Clin Cancer Res. 2022. PMID: 35583609 Free PMC article. Review.
-
BRAF and MEK Inhibitors Affect Dendritic-Cell Maturation and T-Cell Stimulation.Int J Mol Sci. 2021 Nov 4;22(21):11951. doi: 10.3390/ijms222111951. Int J Mol Sci. 2021. PMID: 34769379 Free PMC article.
References
-
- Burnet F.M. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970;13:1–27. - PubMed
-
- Thomas L. Discussion. In: Lawrence H.S., editor. Cellular and Humoral Aspects of the Hypersensitive States. Hoeber-Harper; New York, NY, USA: 1959.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

