Biology and clinical relevance of EpCAM
- PMID: 31225512
- PMCID: PMC6558934
- DOI: 10.15698/cst2019.06.188
Biology and clinical relevance of EpCAM
Abstract
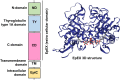
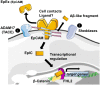
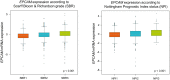
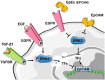
Epithelial cell adhesion molecule (EpCAM) is a transmembrane glycoprotein primarily known to mediate homotypic cell contacts in epithelia tissues. Because EpCAM expression is limited to normal and malignant epithelia, it has been used as diagnostic marker for the detection of carcinoma cells in mesenchymal organs such as blood, bone marrow or lymph nodes. In particular, the detection and molecular characterization of EpCAM-positive circulating tumor cells (CTCs) in the blood of carcinoma patients has gained considerable interest over the past ten years. EpCAM is primarily considered as an adhesion molecule, but recent studies have shown diverse biological functions including regulation of cell proliferation and cancer stemness. In this review, we summarize the current knowledge on the biological properties of EpCAM with emphasis on mechanisms involved in cancer progression and discuss the clinical implications of these findings for the clinical use of EpCAM as a diagnostic marker.
Keywords: EMT; EpCAM; Tumor biomarker; circulating tumor cells; liquid biopsy; tumor cell dissemination.
Conflict of interest statement
Conflict of interest: K.P. has ongoing patent applications related to circulating tumor cells. K.P. has received honoraria from Agena, Novartis, Roche and Sanofi and research funding from European Federation of Pharmaceutical Industries and Associations (EFPIA) partners (Angle, Menarini and Servier) of the CANCER-ID programme of the European Union–EFPIA Innovative Medicines Initiative. L.K and S.W. declare no conflict of interest.
Figures
Similar articles
-
Expression and function of epithelial cell adhesion molecule EpCAM: where are we after 40 years?Cancer Metastasis Rev. 2020 Sep;39(3):969-987. doi: 10.1007/s10555-020-09898-3. Cancer Metastasis Rev. 2020. PMID: 32507912 Free PMC article. Review.
-
Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule.Am J Pathol. 2007 Aug;171(2):386-95. doi: 10.2353/ajpath.2007.070152. Epub 2007 Jun 28. Am J Pathol. 2007. PMID: 17600130 Free PMC article. Review.
-
Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer.Oncotarget. 2016 Apr 26;7(17):24677-87. doi: 10.18632/oncotarget.8250. Oncotarget. 2016. PMID: 27013581 Free PMC article.
-
Circulating tumour cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research.Ann Oncol. 2014 Aug;25(8):1506-16. doi: 10.1093/annonc/mdu018. Epub 2014 Mar 20. Ann Oncol. 2014. PMID: 24651410 Review.
-
Circulating Tumor Cells: A Review of Non-EpCAM-Based Approaches for Cell Enrichment and Isolation.Clin Chem. 2016 Apr;62(4):571-81. doi: 10.1373/clinchem.2015.249706. Epub 2016 Feb 19. Clin Chem. 2016. PMID: 26896446 Review.
Cited by
-
Differentiation of Cells Isolated from Afterbirth Tissues into Hepatocyte-Like Cells and Their Potential Clinical Application in Liver Regeneration.Stem Cell Rev Rep. 2021 Apr;17(2):581-603. doi: 10.1007/s12015-020-10045-2. Epub 2020 Sep 25. Stem Cell Rev Rep. 2021. PMID: 32974851 Free PMC article. Review.
-
Clinical Progresses and Challenges of Bispecific Antibodies for the Treatment of Solid Tumors.Mol Diagn Ther. 2024 Nov;28(6):669-702. doi: 10.1007/s40291-024-00734-w. Epub 2024 Aug 22. Mol Diagn Ther. 2024. PMID: 39172329 Free PMC article. Review.
-
Extracellular Vesicles Released by Enterovirus-Infected EndoC-βH1 Cells Mediate Non-Lytic Viral Spread.Microorganisms. 2020 Nov 8;8(11):1753. doi: 10.3390/microorganisms8111753. Microorganisms. 2020. PMID: 33171580 Free PMC article.
-
Liquid biopsy: from discovery to clinical implementation.Mol Oncol. 2021 Jun;15(6):1617-1621. doi: 10.1002/1878-0261.12997. Mol Oncol. 2021. PMID: 34075709 Free PMC article. No abstract available.
-
Circulating tumor cells in pancreatic cancer: more than liquid biopsy.Ther Adv Med Oncol. 2024 Oct 9;16:17588359241284935. doi: 10.1177/17588359241284935. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39421679 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
