Cancer DNA vaccines: current preclinical and clinical developments and future perspectives
- PMID: 30953535
- PMCID: PMC6449928
- DOI: 10.1186/s13046-019-1154-7
Cancer DNA vaccines: current preclinical and clinical developments and future perspectives
Abstract
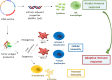
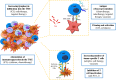
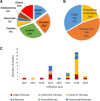
The recent developments in immuno-oncology have opened an unprecedented avenue for the emergence of vaccine strategies. Therapeutic DNA cancer vaccines are now considered a very promising strategy to activate the immune system against cancer. In the past, several clinical trials using plasmid DNA vaccines demonstrated a good safety profile and the activation of a broad and specific immune response. However, these vaccines often demonstrated only modest therapeutic effects in clinical trials due to the immunosuppressive mechanisms developed by the tumor. To enhance the vaccine-induced immune response and the treatment efficacy, DNA vaccines could be improved by using two different strategies. The first is to increase their immunogenicity by selecting and optimizing the best antigen(s) to be inserted into the plasmid DNA. The second strategy is to combine DNA vaccines with other complementary therapies that could improve their activity by attenuating immunosuppression in the tumor microenvironment or by increasing the activity/number of immune cells. A growing number of preclinical and clinical studies are adopting these two strategies to better exploit the potential of DNA vaccination. In this review, we analyze the last 5-year preclinical studies and 10-year clinical trials using plasmid DNA vaccines for cancer therapy. We also investigate the strategies that are being developed to overcome the limitations in cancer DNA vaccination, revisiting the rationale for different combinations of therapy and the different possibilities in antigen choice. Finally, we highlight the most promising developments and critical points that need to be addressed to move towards the approval of therapeutic cancer DNA vaccines as part of the standard of cancer care in the future.
Keywords: Antigens; Cancer; Cancer vaccines; Combination therapy; DNA vaccines; Immuno-oncology; Immunotherapy; Neoantigens.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
The future of cancer immunotherapy: DNA vaccines leading the way.Med Oncol. 2023 Jun 9;40(7):200. doi: 10.1007/s12032-023-02060-3. Med Oncol. 2023. PMID: 37294501 Free PMC article. Review.
-
DNA vaccines to attack cancer: Strategies for improving immunogenicity and efficacy.Pharmacol Ther. 2016 Sep;165:32-49. doi: 10.1016/j.pharmthera.2016.05.004. Epub 2016 May 24. Pharmacol Ther. 2016. PMID: 27235391 Review.
-
DNA Vaccines to Improve Immunogenicity and Effectiveness in Cancer Vaccinations: Advancement and Developments.Curr Gene Ther. 2023;23(3):170-183. doi: 10.2174/1566523223666221219094849. Curr Gene Ther. 2023. PMID: 36537599
-
DNA vaccine for cancer immunotherapy.Hum Vaccin Immunother. 2014;10(11):3153-64. doi: 10.4161/21645515.2014.980686. Hum Vaccin Immunother. 2014. PMID: 25625927 Free PMC article. Review.
-
Therapeutic cancer vaccines: past, present, and future.Adv Cancer Res. 2013;119:421-75. doi: 10.1016/B978-0-12-407190-2.00007-1. Adv Cancer Res. 2013. PMID: 23870514 Free PMC article. Review.
Cited by
-
Neoantigen vaccine platforms in clinical development: understanding the future of personalized immunotherapy.Expert Opin Investig Drugs. 2021 May;30(5):529-541. doi: 10.1080/13543784.2021.1896702. Epub 2021 Mar 31. Expert Opin Investig Drugs. 2021. PMID: 33641576 Free PMC article. Review.
-
Advances in Lipid-Based Nanoparticles for Cancer Chemoimmunotherapy.Pharmaceutics. 2021 Apr 9;13(4):520. doi: 10.3390/pharmaceutics13040520. Pharmaceutics. 2021. PMID: 33918635 Free PMC article. Review.
-
Transcriptional Targeting of Dendritic Cells Using an Optimized Human Fascin1 Gene Promoter.Int J Mol Sci. 2023 Nov 29;24(23):16938. doi: 10.3390/ijms242316938. Int J Mol Sci. 2023. PMID: 38069260 Free PMC article.
-
From Conventional to Precision Therapy in Canine Mammary Cancer: A Comprehensive Review.Front Vet Sci. 2021 Feb 17;8:623800. doi: 10.3389/fvets.2021.623800. eCollection 2021. Front Vet Sci. 2021. PMID: 33681329 Free PMC article. Review.
-
A novel intranasal peptide vaccine inhibits non-small cell lung cancer with KRAS mutation.Cancer Gene Ther. 2024 Mar;31(3):464-471. doi: 10.1038/s41417-023-00717-9. Epub 2024 Jan 4. Cancer Gene Ther. 2024. PMID: 38177307
References
-
- Gasser M, Waaga-Gasser AM. Therapeutic antibodies in Cancer therapy. In: Böldicke T, editor. Protein targeting compounds: prediction, selection and activity of specific inhibitors. Cham: Springer International Publishing; 2016. pp. 95–120.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

