Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia
- PMID: 29880584
- PMCID: PMC6385599
- DOI: 10.1158/2159-8290.CD-17-1319
Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia
Abstract
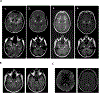
CD19-specific chimeric antigen receptor (CAR) T-cell therapy is highly effective against relapsed or refractory acute lymphoblastic leukemia (ALL), but is hindered by neurotoxicity. In 53 adult patients with ALL, we found a significant association of severe neurotoxicity with high pretreatment disease burden, higher peak CAR T-cell expansion, and early and higher elevations of proinflammatory cytokines in blood. Patients with severe neurotoxicity had evidence of blood-cerebrospinal fluid (CSF) barrier disruption correlating with neurotoxicity grade without association with CSF white blood cell count or CAR T-cell quantity in CSF. Proinflammatory cytokines were enriched in CSF during severe neurotoxicity with disproportionately high levels of IL6, IL8, MCP1, and IP10, suggesting central nervous system-specific production. Seizures, seizure-like activity, myoclonus, and neuroimaging characteristics suggested excitatory neurotoxicity, and we found elevated levels of endogenous excitatory agonists in CSF during neurotoxicity.Significance: We detail the neurologic symptoms and blood, CSF, and neuroimaging correlates of neurotoxicity associated with CD19 CAR T cells and identify neurotoxicity risk factors. Our findings implicate cellular components other than T cells and suggest novel links between systemic inflammation and characteristic neurotoxicity symptoms. Cancer Discov; 8(8); 958-71. ©2018 AACR.This article is highlighted in the In This Issue feature, p. 899.
©2018 American Association for Cancer Research.
Figures



Similar articles
-
Toxicity and effectiveness of CD19 CAR T therapy in children with high-burden central nervous system refractory B-ALL.Cancer Immunol Immunother. 2021 Jul;70(7):1979-1993. doi: 10.1007/s00262-020-02829-9. Epub 2021 Jan 8. Cancer Immunol Immunother. 2021. PMID: 33416942 Free PMC article.
-
Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy.Blood. 2019 Dec 12;134(24):2149-2158. doi: 10.1182/blood.2019001463. Blood. 2019. PMID: 31697826 Free PMC article.
-
Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL.Blood. 2019 Dec 26;134(26):2361-2368. doi: 10.1182/blood.2019001641. Blood. 2019. PMID: 31650176 Free PMC article. Clinical Trial.
-
Chimeric Antigen Receptor Therapy in Acute Lymphoblastic Leukemia Clinical Practice.Curr Hematol Malig Rep. 2017 Aug;12(4):370-379. doi: 10.1007/s11899-017-0394-x. Curr Hematol Malig Rep. 2017. PMID: 28656487 Review.
-
Paving the road ahead for CD19 CAR T-cell therapy.Curr Opin Hematol. 2015 Nov;22(6):516-20. doi: 10.1097/MOH.0000000000000182. Curr Opin Hematol. 2015. PMID: 26335422 Free PMC article. Review.
Cited by
-
Therapeutic potential of EVs loaded with CB2 receptor agonist in spinal cord injury via the Nrf2/HO-1 pathway.Redox Rep. 2024 Dec;29(1):2420572. doi: 10.1080/13510002.2024.2420572. Epub 2024 Oct 28. Redox Rep. 2024. PMID: 39466990 Free PMC article.
-
Neurotoxicity and Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy: Insights Into Mechanisms and Novel Therapies.Front Immunol. 2020 Aug 28;11:1973. doi: 10.3389/fimmu.2020.01973. eCollection 2020. Front Immunol. 2020. PMID: 32983132 Free PMC article. Review.
-
Cytokine release syndrome-associated encephalopathy in patients with COVID-19.Eur J Neurol. 2021 Jan;28(1):248-258. doi: 10.1111/ene.14491. Epub 2020 Oct 5. Eur J Neurol. 2021. PMID: 32853434 Free PMC article.
-
EEG-based grading of immune effector cell-associated neurotoxicity syndrome.Sci Rep. 2022 Nov 21;12(1):20011. doi: 10.1038/s41598-022-24010-1. Sci Rep. 2022. PMID: 36414694 Free PMC article.
-
Endothelial activation and damage as a common pathological substrate in different pathologies and cell therapy complications.Front Med (Lausanne). 2023 Nov 14;10:1285898. doi: 10.3389/fmed.2023.1285898. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38034541 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

