Brain-computer interfaces for post-stroke motor rehabilitation: a meta-analysis
- PMID: 29761128
- PMCID: PMC5945970
- DOI: 10.1002/acn3.544
Brain-computer interfaces for post-stroke motor rehabilitation: a meta-analysis
Abstract
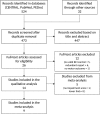
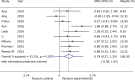
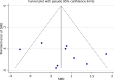
Brain-computer interfaces (BCIs) can provide sensory feedback of ongoing brain oscillations, enabling stroke survivors to modulate their sensorimotor rhythms purposefully. A number of recent clinical studies indicate that repeated use of such BCIs might trigger neurological recovery and hence improvement in motor function. Here, we provide a first meta-analysis evaluating the clinical effectiveness of BCI-based post-stroke motor rehabilitation. Trials were identified using MEDLINE, CENTRAL, PEDro and by inspection of references in several review articles. We selected randomized controlled trials that used BCIs for post-stroke motor rehabilitation and provided motor impairment scores before and after the intervention. A random-effects inverse variance method was used to calculate the summary effect size. We initially identified 524 articles and, after removing duplicates, we screened titles and abstracts of 473 articles. We found 26 articles corresponding to BCI clinical trials, of these, there were nine studies that involved a total of 235 post-stroke survivors that fulfilled the inclusion criterion (randomized controlled trials that examined motor performance as an outcome measure) for the meta-analysis. Motor improvements, mostly quantified by the upper limb Fugl-Meyer Assessment (FMA-UE), exceeded the minimal clinically important difference (MCID=5.25) in six BCI studies, while such improvement was reached only in three control groups. Overall, the BCI training was associated with a standardized mean difference of 0.79 (95% CI: 0.37 to 1.20) in FMA-UE compared to control conditions, which is in the range of medium to large summary effect size. In addition, several studies indicated BCI-induced functional and structural neuroplasticity at a subclinical level. This suggests that BCI technology could be an effective intervention for post-stroke upper limb rehabilitation. However, more studies with larger sample size are required to increase the reliability of these results.
Figures


Similar articles
-
The clinical effects of brain-computer interface with robot on upper-limb function for post-stroke rehabilitation: a meta-analysis and systematic review.Disabil Rehabil Assist Technol. 2024 Jan;19(1):30-41. doi: 10.1080/17483107.2022.2060354. Epub 2022 Apr 21. Disabil Rehabil Assist Technol. 2024. PMID: 35450498
-
The Promotoer, a brain-computer interface-assisted intervention to promote upper limb functional motor recovery after stroke: a study protocol for a randomized controlled trial to test early and long-term efficacy and to identify determinants of response.BMC Neurol. 2020 Jun 27;20(1):254. doi: 10.1186/s12883-020-01826-w. BMC Neurol. 2020. PMID: 32593293 Free PMC article.
-
Immediate and long-term effects of BCI-based rehabilitation of the upper extremity after stroke: a systematic review and meta-analysis.J Neuroeng Rehabil. 2020 Apr 25;17(1):57. doi: 10.1186/s12984-020-00686-2. J Neuroeng Rehabil. 2020. PMID: 32334608 Free PMC article.
-
Brain-Computer Interface Training Based on Brain Activity Can Induce Motor Recovery in Patients With Stroke: A Meta-Analysis.Neurorehabil Neural Repair. 2022 Feb;36(2):83-96. doi: 10.1177/15459683211062895. Epub 2021 Dec 27. Neurorehabil Neural Repair. 2022. PMID: 34958261 Review.
-
Why we should systematically assess, control and report somatosensory impairments in BCI-based motor rehabilitation after stroke studies.Neuroimage Clin. 2020;28:102417. doi: 10.1016/j.nicl.2020.102417. Epub 2020 Sep 15. Neuroimage Clin. 2020. PMID: 33039972 Free PMC article. Review.
Cited by
-
fNIRS-EEG BCIs for Motor Rehabilitation: A Review.Bioengineering (Basel). 2023 Dec 6;10(12):1393. doi: 10.3390/bioengineering10121393. Bioengineering (Basel). 2023. PMID: 38135985 Free PMC article. Review.
-
Decoding of Ankle Joint Movements in Stroke Patients Using Surface Electromyography.Sensors (Basel). 2021 Feb 24;21(5):1575. doi: 10.3390/s21051575. Sensors (Basel). 2021. PMID: 33668229 Free PMC article.
-
Easily Attach/Detach Reattachable EEG Headset with Candle-like Microneedle Electrodes.Micromachines (Basel). 2023 Feb 6;14(2):400. doi: 10.3390/mi14020400. Micromachines (Basel). 2023. PMID: 36838100 Free PMC article.
-
Brain-machine interface-based training for improving upper extremity function after stroke: A meta-analysis of randomized controlled trials.Front Neurosci. 2022 Aug 3;16:949575. doi: 10.3389/fnins.2022.949575. eCollection 2022. Front Neurosci. 2022. PMID: 35992923 Free PMC article.
-
"Mine Works Better": Examining the Influence of Embodiment in Virtual Reality on the Sense of Agency During a Binary Motor Imagery Task With a Brain-Computer Interface.Front Psychol. 2021 Dec 24;12:806424. doi: 10.3389/fpsyg.2021.806424. eCollection 2021. Front Psychol. 2021. PMID: 35002899 Free PMC article.
References
-
- Organization WH . 2014. The top 10 causes of death. July 2013. Available Who Intmediacentrefactsheetsfs310en. Last Accessed July 2014.
-
- Association S. State of the Nation, Stroke Statistics . 2016; Available from: https://www.stroke.org.uk/sites/default/files/stroke_statistics_2015.pdf
-
- Lawrence ES, Coshall C, Dundas R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 2001;32:1279–1284. - PubMed
-
- Lang CE, Beebe JA. Relating movement control at 9 upper extremity segments to loss of hand function in people with chronic hemiparesis. Neurorehabil Neural Repair 2007;21:279–291. - PubMed
-
- Taub E, Uswatte G, Pidikiti R. Constraint‐induced movement therapy: a new family of techniques with broad application to physical rehabilitation–a clinical review. J Rehabil Res Dev 1999;36:237. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

