Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo
- PMID: 28284008
- PMCID: PMC6160382
- DOI: 10.1007/s13238-017-0384-8
Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo
Abstract
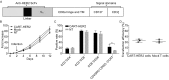
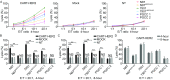
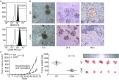
Human epidermal growth factor receptor 2 (HER2) proteins are overexpressed in a high proportion of gastric cancer (GC) cases and affect the maintenance of cancer stem cell (CSC) subpopulations, which are used as targets for the clinical treatment of patients with HER2-positive GC. Despite improvements in survival, numerous HER2-positive patients fail treatment with trastuzumab, highlighting the need for more effective therapies. In this study, we generated a novel type of genetically modified human T cells, expressing a chimeric antigen receptor (CAR), and targeting the GC cell antigen HER2, which harbors the CD137 and CD3ζ moieties. Our findings show that the expanded CAR-T cells, expressing an increased central memory phenotype, were activated by the specific recognition of HER2 antigens in an MHC-independent manner, and effectively killed patient-derived HER2-positive GC cells. In HER2-positive xenograft tumors, CAR-T cells exhibited considerably enhanced tumor inhibition ability, long-term survival, and homing to targets, compared with those of non-transduced T cells. The sphere-forming ability and in vivo tumorigenicity of patient-derived gastric cancer stem-like cells, expressing HER2 and the CD44 protein, were also inhibited. Our results support the future development and clinical application of this adoptive immunotherapy in patients with HER2-positive advanced GC.
Keywords: CD137; HER2; cancer stem cell; chimeric antigen receptor; gastric cancer; immunotherapy.
Figures


Similar articles
-
Construction and evaluation of a novel humanized HER2-specific chimeric receptor.Breast Cancer Res. 2014 Jun 11;16(3):R61. doi: 10.1186/bcr3674. Breast Cancer Res. 2014. PMID: 24919843 Free PMC article.
-
Development of chimeric antigen receptor-modified T cells for the treatment of esophageal cancer.Tumori. 2021 Aug;107(4):341-352. doi: 10.1177/0300891620960223. Epub 2020 Sep 28. Tumori. 2021. PMID: 32988314
-
HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors.Clin Cancer Res. 2010 Jan 15;16(2):474-85. doi: 10.1158/1078-0432.CCR-09-1322. Epub 2010 Jan 12. Clin Cancer Res. 2010. PMID: 20068073 Free PMC article.
-
Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor (CAR) for tumor immunotherapy; recent progress.Stem Cell Res Ther. 2022 Jan 29;13(1):40. doi: 10.1186/s13287-022-02719-0. Stem Cell Res Ther. 2022. PMID: 35093187 Free PMC article. Review.
-
Chimeric antigen receptor T cells: a novel therapy for solid tumors.J Hematol Oncol. 2017 Mar 29;10(1):78. doi: 10.1186/s13045-017-0444-9. J Hematol Oncol. 2017. PMID: 28356156 Free PMC article. Review.
Cited by
-
CD19-CAR-T Cells Bearing a KIR/PD-1-Based Inhibitory CAR Eradicate CD19+HLA-C1- Malignant B Cells While Sparing CD19+HLA-C1+ Healthy B Cells.Cancers (Basel). 2020 Sep 13;12(9):2612. doi: 10.3390/cancers12092612. Cancers (Basel). 2020. PMID: 32933182 Free PMC article.
-
Advances in CAR T-cell therapy in bile duct, pancreatic, and gastric cancers.Front Immunol. 2022 Oct 6;13:1025608. doi: 10.3389/fimmu.2022.1025608. eCollection 2022. Front Immunol. 2022. PMID: 36341440 Free PMC article. Review.
-
A promising antitumor method: Targeting CSC with immune cells modified with CAR.Front Immunol. 2022 Aug 11;13:937327. doi: 10.3389/fimmu.2022.937327. eCollection 2022. Front Immunol. 2022. PMID: 36032145 Free PMC article. Review.
-
Chimeric Antigen Receptor-T Cell and Oncolytic Viral Therapies for Gastric Cancer and Peritoneal Carcinomatosis of Gastric Origin: Path to Improving Combination Strategies.Cancers (Basel). 2023 Nov 30;15(23):5661. doi: 10.3390/cancers15235661. Cancers (Basel). 2023. PMID: 38067366 Free PMC article. Review.
-
Research progress on the immune microenvironment and immunotherapy in gastric cancer.Front Immunol. 2023 Nov 23;14:1291117. doi: 10.3389/fimmu.2023.1291117. eCollection 2023. Front Immunol. 2023. PMID: 38077373 Free PMC article. Review.
References
-
- Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, et al. Human Epidermal Growth Factor Receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

