Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single and multicenter trials
- PMID: 27580889
- PMCID: PMC5193027
- DOI: 10.1093/neuonc/now187
Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single and multicenter trials
Abstract
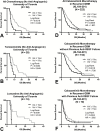
Background: The prognostic significance of baseline contrast enhancing tumor prior to second- or third-line therapy in recurrent glioblastoma (GBM) for overall survival (OS) remains controversial, particularly in the context of repeated surgical resection and/or use of anti-angiogenic therapy. In the current study, we examined recurrent GBM patients from both single and multicenter clinical trials to test whether baseline enhancing tumor volume, including central necrosis, is a significant prognostic factor for OS in recurrent GBM.
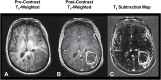
Methods: Included were 497 patients with recurrent GBM from 4 data sources: 2 single-center sites (University of Toronto, University of California Los Angeles) and 2 phase II multicenter trials (AVF3708G, Bevacizumab ± Irinotecan, NCT00345163; XL184-201, Cabozantinib, NCT00704288). T1 subtraction maps were used to define volume of contrast enhancing tumor, including central necrosis. Cox multivariable and univariate analyses were used to evaluate the relationship between tumor volume prior to second- or third-line therapy and OS.
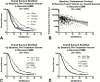
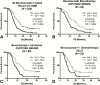
Results: Both continuous measures of baseline tumor volume and tumors dichotomized into large (≥15cc) and small (<15cc) tumors were significant predictors of OS (P<.0001), independently of age and treatment. Univariate analysis demonstrated significant OS differences (P<.05) between large (≥15cc) and small (<15cc) tumors in patients under all therapeutic scenarios. Only patients treated with cabozantinib who previously failed anti-angiogenic therapy did not show an OS dependence on baseline tumor volume.
Conclusions: Baseline tumor volume is a significant prognostic factor in recurrent GBM. Clinical trial treatment arms must have a balanced distribution of tumor size, and tumor size should be considered when interpreting therapeutic efficacy.
Keywords: T1 subtraction; bevacizumab; cabozantinib; glioblastoma; recurrent; tumor volume.
© The Author(s) 2016. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Where size matters: imaging-based biomarkers for patient stratification.Neuro Oncol. 2017 Jan;19(1):7-8. doi: 10.1093/neuonc/now248. Neuro Oncol. 2017. PMID: 28031381 Free PMC article. No abstract available.
Similar articles
-
Volumetric response quantified using T1 subtraction predicts long-term survival benefit from cabozantinib monotherapy in recurrent glioblastoma.Neuro Oncol. 2018 Sep 3;20(10):1411-1418. doi: 10.1093/neuonc/noy054. Neuro Oncol. 2018. PMID: 29660005 Free PMC article. Clinical Trial.
-
Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 Central Reader Study.Neuro Oncol. 2013 Jul;15(7):945-54. doi: 10.1093/neuonc/not049. Epub 2013 Jun 19. Neuro Oncol. 2013. PMID: 23788270 Free PMC article. Clinical Trial.
-
Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial.Radiology. 2014 Apr;271(1):200-10. doi: 10.1148/radiol.13131305. Epub 2013 Nov 27. Radiology. 2014. PMID: 24475840 Free PMC article. Clinical Trial.
-
Bevacizumab: a treatment option for recurrent glioblastoma multiforme.Ann Pharmacother. 2008 Oct;42(10):1486-90. doi: 10.1345/aph.1L030. Epub 2008 Sep 2. Ann Pharmacother. 2008. PMID: 18765835 Review.
-
Biomarkers in Recurrent Grade III Glioma Patients Treated with Bevacizumab and Irinotecan.Cancer Invest. 2018 Feb 7;36(2):165-174. doi: 10.1080/07357907.2018.1430818. Epub 2018 Feb 2. Cancer Invest. 2018. PMID: 29393706 Review.
Cited by
-
Synthesizing a Contrast-Enhancement Map in Patients with High-Grade Gliomas Based on a Postcontrast MR Imaging Quantification Only.AJNR Am J Neuroradiol. 2018 Dec;39(12):2194-2199. doi: 10.3174/ajnr.A5870. Epub 2018 Nov 8. AJNR Am J Neuroradiol. 2018. PMID: 30409854 Free PMC article.
-
Incidence, molecular characteristics, and imaging features of "clinically-defined pseudoprogression" in newly diagnosed glioblastoma treated with chemoradiation.J Neurooncol. 2022 Sep;159(3):509-518. doi: 10.1007/s11060-022-04088-3. Epub 2022 Jul 17. J Neurooncol. 2022. PMID: 35842871
-
Localized Blood-Brain Barrier Opening in Ovine Model Using Image-Guided Transcranial Focused Ultrasound.Ultrasound Med Biol. 2019 Sep;45(9):2391-2404. doi: 10.1016/j.ultrasmedbio.2019.05.023. Epub 2019 Jun 17. Ultrasound Med Biol. 2019. PMID: 31217090 Free PMC article.
-
18F-FDOPA PET and MRI characteristics correlate with degree of malignancy and predict survival in treatment-naïve gliomas: a cross-sectional study.J Neurooncol. 2018 Sep;139(2):399-409. doi: 10.1007/s11060-018-2877-6. Epub 2018 Apr 20. J Neurooncol. 2018. PMID: 29679199 Free PMC article.
-
A radiomics nomogram based on multiparametric MRI might stratify glioblastoma patients according to survival.Eur Radiol. 2019 Oct;29(10):5528-5538. doi: 10.1007/s00330-019-06069-z. Epub 2019 Mar 7. Eur Radiol. 2019. PMID: 30847586
References
-
- Kelly PJ, Daumas-Duport C, Scheithauer BW, et al. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987;62(6):450–459. - PubMed
-
- Kelly PJ, Daumas-Duport C, Kispert DB, et al. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66(6):865–874. - PubMed
-
- Earnest Ft, Kelly PJ, Scheithauer BW, et al. Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology. 1988;166(3):823–827. - PubMed
-
- Vogelbaum MA. Does extent of resection of a glioblastoma matter? Clin Neurosurg. 2012;59:79–81. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

