An algorithm to predict the connectome of neural microcircuits
- PMID: 26500529
- PMCID: PMC4597796
- DOI: 10.3389/fncom.2015.00120
An algorithm to predict the connectome of neural microcircuits
Abstract
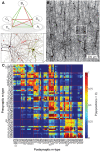
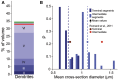
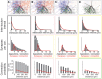
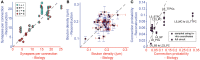
Experimentally mapping synaptic connections, in terms of the numbers and locations of their synapses and estimating connection probabilities, is still not a tractable task, even for small volumes of tissue. In fact, the six layers of the neocortex contain thousands of unique types of synaptic connections between the many different types of neurons, of which only a handful have been characterized experimentally. Here we present a theoretical framework and a data-driven algorithmic strategy to digitally reconstruct the complete synaptic connectivity between the different types of neurons in a small well-defined volume of tissue-the micro-scale connectome of a neural microcircuit. By enforcing a set of established principles of synaptic connectivity, and leveraging interdependencies between fundamental properties of neural microcircuits to constrain the reconstructed connectivity, the algorithm yields three parameters per connection type that predict the anatomy of all types of biologically viable synaptic connections. The predictions reproduce a spectrum of experimental data on synaptic connectivity not used by the algorithm. We conclude that an algorithmic approach to the connectome can serve as a tool to accelerate experimental mapping, indicating the minimal dataset required to make useful predictions, identifying the datasets required to improve their accuracy, testing the feasibility of experimental measurements, and making it possible to test hypotheses of synaptic connectivity.
Keywords: algorithm development; connectome mapping; cortical circuits; in silico; neocortex; somatosensory cortex; synaptic transmission.
Figures


Similar articles
-
Data-Driven Modeling of Cholinergic Modulation of Neural Microcircuits: Bridging Neurons, Synapses and Network Activity.Front Neural Circuits. 2018 Oct 9;12:77. doi: 10.3389/fncir.2018.00077. eCollection 2018. Front Neural Circuits. 2018. PMID: 30356701 Free PMC article.
-
Statistical connectivity provides a sufficient foundation for specific functional connectivity in neocortical neural microcircuits.Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):E2885-94. doi: 10.1073/pnas.1202128109. Epub 2012 Sep 18. Proc Natl Acad Sci U S A. 2012. PMID: 22991468 Free PMC article.
-
Predicting the Dynamics of Network Connectivity in the Neocortex.J Neurosci. 2015 Sep 9;35(36):12535-44. doi: 10.1523/JNEUROSCI.2917-14.2015. J Neurosci. 2015. PMID: 26354919 Free PMC article.
-
Mapping functional connectivity in barrel-related columns reveals layer- and cell type-specific microcircuits.Brain Struct Funct. 2007 Sep;212(2):107-19. doi: 10.1007/s00429-007-0147-z. Epub 2007 Jun 26. Brain Struct Funct. 2007. PMID: 17717691 Review.
-
Synaptic pathways in neural microcircuits.Trends Neurosci. 2005 Oct;28(10):541-51. doi: 10.1016/j.tins.2005.08.004. Trends Neurosci. 2005. PMID: 16122815 Review.
Cited by
-
Data-Driven Modeling of Cholinergic Modulation of Neural Microcircuits: Bridging Neurons, Synapses and Network Activity.Front Neural Circuits. 2018 Oct 9;12:77. doi: 10.3389/fncir.2018.00077. eCollection 2018. Front Neural Circuits. 2018. PMID: 30356701 Free PMC article.
-
Multimodal Modeling of Neural Network Activity: Computing LFP, ECoG, EEG, and MEG Signals With LFPy 2.0.Front Neuroinform. 2018 Dec 18;12:92. doi: 10.3389/fninf.2018.00092. eCollection 2018. Front Neuroinform. 2018. PMID: 30618697 Free PMC article.
-
A realistic morpho-anatomical connection strategy for modelling full-scale point-neuron microcircuits.Sci Rep. 2022 Aug 16;12(1):13864. doi: 10.1038/s41598-022-18024-y. Sci Rep. 2022. PMID: 35974119 Free PMC article.
-
Comments and General Discussion on "The Anatomical Problem Posed by Brain Complexity and Size: A Potential Solution".Front Neuroanat. 2016 Jun 10;10:60. doi: 10.3389/fnana.2016.00060. eCollection 2016. Front Neuroanat. 2016. PMID: 27375436 Free PMC article. No abstract available.
-
Connectivity concepts in neuronal network modeling.PLoS Comput Biol. 2022 Sep 8;18(9):e1010086. doi: 10.1371/journal.pcbi.1010086. eCollection 2022 Sep. PLoS Comput Biol. 2022. PMID: 36074778 Free PMC article. Review.
References
-
- Beierlein M., Connors B. W. (2002). Short-term dynamics of thalamocortical and intracortical synapses onto layer 6 neurons in neocortex. J. Neurophysiol. 88, 1924–1932. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

