Embryonic Medaka Model of Microglia in the Developing CNS Allowing In Vivo Analysis of Their Spatiotemporal Recruitment in Response to Irradiation
- PMID: 26061282
- PMCID: PMC4465025
- DOI: 10.1371/journal.pone.0127325
Embryonic Medaka Model of Microglia in the Developing CNS Allowing In Vivo Analysis of Their Spatiotemporal Recruitment in Response to Irradiation
Abstract
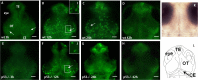
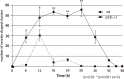
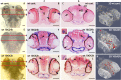
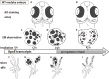
Radiation therapy (RT) is pivotal in the treatment of many central nervous system (CNS) pathologies; however, exposure to RT in children is associated with a higher risk of secondary CNS tumors. Although recent research interest has focused on the reparative and therapeutic role of microglia, their recruitment following RT has not been elucidated, especially in the developing CNS. Here, we investigated the spatiotemporal dynamics of microglia during tissue repair in the irradiated embryonic medaka brain by whole-mount in situ hybridization using a probe for Apolipoprotein E (ApoE), a marker for activated microglia in teleosts. Three-dimensional imaging of the distribution of ApoE-expressing microglia in the irradiated embryonic brain clearly showed that ApoE-expressing microglia were abundant only in the late phase of phagocytosis during tissue repair induced by irradiation, while few microglia expressed ApoE in the initial phase of phagocytosis. This strongly suggests that ApoE has a significant function in the late phase of phagocytosis by microglia in the medaka brain. In addition, the distribution of microglia in p53-deficient embryos at the late phase of phagocytosis was almost the same as in wild-type embryos, despite the low numbers of irradiation-induced apoptotic neurons, suggesting that constant numbers of activated microglia were recruited at the late phase of phagocytosis irrespective of the extent of neuronal injury. This medaka model of microglia demonstrated specific recruitment after irradiation in the developing CNS and could provide a useful potential therapeutic strategy to counteract the detrimental effects of RT.
Conflict of interest statement
Figures

Similar articles
-
Radical change of apoptotic strategy following irradiation during later period of embryogenesis in medaka (Oryzias latipes).PLoS One. 2018 Aug 3;13(8):e0201790. doi: 10.1371/journal.pone.0201790. eCollection 2018. PLoS One. 2018. PMID: 30075024 Free PMC article.
-
Abscopal Activation of Microglia in Embryonic Fish Brain Following Targeted Irradiation with Heavy-Ion Microbeam.Int J Mol Sci. 2017 Jul 4;18(7):1428. doi: 10.3390/ijms18071428. Int J Mol Sci. 2017. PMID: 28677658 Free PMC article.
-
Irradiation-injured brain tissues can self-renew in the absence of the pivotal tumor suppressor p53 in the medaka (Oryzias latipes) embryo.J Radiat Res. 2016 Jan;57(1):9-15. doi: 10.1093/jrr/rrv054. Epub 2015 Sep 25. J Radiat Res. 2016. PMID: 26410759 Free PMC article.
-
Microglia in central nervous system repair after injury.J Biochem. 2016 May;159(5):491-6. doi: 10.1093/jb/mvw009. Epub 2016 Feb 8. J Biochem. 2016. PMID: 26861995 Review.
-
Role of microglia in embryonic neurogenesis.Exp Biol Med (Maywood). 2016 Sep;241(15):1669-75. doi: 10.1177/1535370216664430. Exp Biol Med (Maywood). 2016. PMID: 27555616 Free PMC article. Review.
Cited by
-
3D reconstructed brain images reveal the possibility of the ogg1 gene to suppress the irradiation-induced apoptosis in embryonic brain in medaka (Oryzias latipes).J Radiat Res. 2022 May 18;63(3):319-330. doi: 10.1093/jrr/rrac005. J Radiat Res. 2022. PMID: 35276012 Free PMC article.
-
Collimated Microbeam Reveals that the Proportion of Non-Damaged Cells in Irradiated Blastoderm Determines the Success of Development in Medaka (Oryzias latipes) Embryos.Biology (Basel). 2020 Dec 5;9(12):447. doi: 10.3390/biology9120447. Biology (Basel). 2020. PMID: 33291358 Free PMC article.
-
Radical change of apoptotic strategy following irradiation during later period of embryogenesis in medaka (Oryzias latipes).PLoS One. 2018 Aug 3;13(8):e0201790. doi: 10.1371/journal.pone.0201790. eCollection 2018. PLoS One. 2018. PMID: 30075024 Free PMC article.
-
Abscopal Activation of Microglia in Embryonic Fish Brain Following Targeted Irradiation with Heavy-Ion Microbeam.Int J Mol Sci. 2017 Jul 4;18(7):1428. doi: 10.3390/ijms18071428. Int J Mol Sci. 2017. PMID: 28677658 Free PMC article.
-
Irradiation-injured brain tissues can self-renew in the absence of the pivotal tumor suppressor p53 in the medaka (Oryzias latipes) embryo.J Radiat Res. 2016 Jan;57(1):9-15. doi: 10.1093/jrr/rrv054. Epub 2015 Sep 25. J Radiat Res. 2016. PMID: 26410759 Free PMC article.
References
-
- Neglia JP, Robison LL, Stovall M, Liu Y, Packer RJ, Hammond S, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98(21): 1528–37. Epub 2006/11/02. doi: 98/21/1528 [pii] 10.1093/jnci/djj411 PubMed . - DOI - PubMed
-
- Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840): 499–505. Epub 2012/06/12. doi: S0140-6736(12)60815-0 [pii] 10.1016/S0140-6736(12)60815-0 PubMed - DOI - PMC - PubMed
-
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726): 1314–8. Epub 2005/04/16. doi: 1110647 [pii] - PubMed
-
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13): 3974–80. Epub 2009/04/03. doi: 29/13/3974 [pii] 10.1523/JNEUROSCI.4363-08.2009 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

