Macrophage-derived chemokine (CCL22) is a novel mediator of lung inflammation following hemorrhage and resuscitation
- PMID: 25136780
- PMCID: PMC4236272
- DOI: 10.1097/SHK.0000000000000253
Macrophage-derived chemokine (CCL22) is a novel mediator of lung inflammation following hemorrhage and resuscitation
Abstract
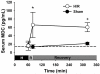
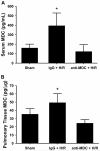
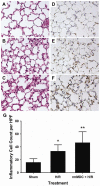
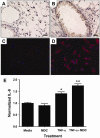
Resuscitation of patients after hemorrhage often results in pulmonary inflammation and places them at risk for the development of acute respiratory distress syndrome. Our previous data indicate that macrophage-derived chemokine (MDC/CCL22) is elevated after resuscitation, but its direct role in this inflammatory response is unknown. Macrophage-derived chemokine signaling through the C-C chemokine receptor type 4 (CCR4) is implicated in other pulmonary proinflammatory conditions, leading us to hypothesize that MDC may also play a role in the pathogenesis of lung inflammation following hemorrhage and resuscitation. To test this, C57BL/6 mice underwent pressure-controlled hemorrhage followed by resuscitation with lactated Ringer's solution. Pulmonary inflammation and inflammatory cell recruitment were analyzed with histological staining, and serum- and tissue-level cytokines were measured by enzyme-linked immunosorbent assay. Pulmonary inflammation and cell recruitment following hemorrhage and resuscitation were associated with systemic MDC levels. Inhibition of MDC via injection of a specific neutralizing antibody prior to hemorrhage and resuscitation significantly reduced pulmonary levels of the chemotactic cytokines keratinocyte-derived chemokine and macrophage inflammatory proteins 2 and 1α, as well as inflammatory cell recruitment to the lungs. Intravenous administration of recombinant MDC prior to resuscitation augmented pulmonary inflammation and cell recruitment. Histological evaluation revealed the expression of CCR4 within the bronchial epithelium, and in vitro treatment of activated bronchial epithelial cells with MDC resulted in production and secretion of neutrophil chemokines. The present study identifies MDC as a novel mediator of lung inflammation after hemorrhage and resuscitation. Macrophage-derived chemokine neutralization may provide a therapeutic strategy to mitigate this inflammatory response.
Figures

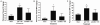
Similar articles
-
IFNγ and TNFα mediate CCL22/MDC production in alveolar macrophages after hemorrhage and resuscitation.Am J Physiol Lung Cell Mol Physiol. 2020 May 1;318(5):L864-L872. doi: 10.1152/ajplung.00455.2019. Epub 2020 Feb 26. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32101016 Free PMC article.
-
Expression of MDC/CCL22 and its receptor CCR4 in rheumatoid arthritis, psoriatic arthritis and osteoarthritis.Cytokine. 2010 Jan;49(1):24-9. doi: 10.1016/j.cyto.2009.10.005. Epub 2009 Nov 25. Cytokine. 2010. PMID: 19942450
-
Immunosuppressive effects of CCL17 on pulmonary antifungal responses during pulmonary invasive aspergillosis.Infect Immun. 2005 Nov;73(11):7198-207. doi: 10.1128/IAI.73.11.7198-7207.2005. Infect Immun. 2005. PMID: 16239514 Free PMC article.
-
Regulation of macrophage-derived chemokine (MDC, CCL22) production.Crit Rev Immunol. 2002;22(2):105-14. Crit Rev Immunol. 2002. PMID: 12433129 Review.
-
Macrophage-derived chemokine (MDC).J Leukoc Biol. 2000 Sep;68(3):400-4. J Leukoc Biol. 2000. PMID: 10985257 Review.
Cited by
-
Multimodal nanoparticle-containing modified suberoylanilide hydroxamic acid polymer conjugates to mitigate immune dysfunction in severe inflammation.Bioeng Transl Med. 2023 Oct 14;9(1):e10611. doi: 10.1002/btm2.10611. eCollection 2024 Jan. Bioeng Transl Med. 2023. PMID: 38193117 Free PMC article.
-
High Abundance of genus Prevotella in the gut of perinatally HIV-infected children is associated with IP-10 levels despite therapy.Sci Rep. 2018 Dec 5;8(1):17679. doi: 10.1038/s41598-018-35877-4. Sci Rep. 2018. PMID: 30518941 Free PMC article.
-
Leukoreduction of packed red blood cells attenuates proinflammatory properties of storage-derived microvesicles.J Surg Res. 2018 Mar;223:128-135. doi: 10.1016/j.jss.2017.09.052. Epub 2017 Dec 22. J Surg Res. 2018. PMID: 29433864 Free PMC article.
-
Predictive value of cytokine/chemokine responses for the disease severity and management in children and adult cases with COVID-19.J Med Virol. 2021 May;93(5):2828-2837. doi: 10.1002/jmv.26683. Epub 2020 Dec 1. J Med Virol. 2021. PMID: 33225509 Free PMC article.
-
IFNγ and TNFα mediate CCL22/MDC production in alveolar macrophages after hemorrhage and resuscitation.Am J Physiol Lung Cell Mol Physiol. 2020 May 1;318(5):L864-L872. doi: 10.1152/ajplung.00455.2019. Epub 2020 Feb 26. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32101016 Free PMC article.
References
-
- Giannoudis PV. Current concepts of the inflammatory response after major trauma: an update. Injury. 2003;34(6):397–404. - PubMed
-
- Hierholzer C, Kelly E, Tsukada K, Loeffert E, Watkins S, Billiar TR, Tweardy DJ. Hemorrhagic shock induces G-CSF expression in bronchial epithelium. Am J Physiol. 1997;273(5 Pt 1):L1058–64. - PubMed
-
- Strieter RM, Kunkel SL. Acute lung injury: the role of cytokines in the elicitation of neutrophils. J Investig Med. 42(4):640–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

