Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain
- PMID: 23921897
- PMCID: PMC3790936
- DOI: 10.1038/jcbfm.2013.128
Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain
Abstract
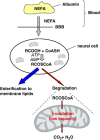
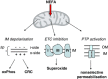
It is puzzling that hydrogen-rich fatty acids are used only poorly as fuel in the brain. The long-standing belief that a slow passage of fatty acids across the blood-brain barrier might be the reason. However, this has been corrected by experimental results. Otherwise, accumulated nonesterified fatty acids or their activated derivatives could exert detrimental activities on mitochondria, which might trigger the mitochondrial route of apoptosis. Here, we draw attention to three particular problems: (1) ATP generation linked to β-oxidation of fatty acids demands more oxygen than glucose, thereby enhancing the risk for neurons to become hypoxic; (2) β-oxidation of fatty acids generates superoxide, which, taken together with the poor anti-oxidative defense in neurons, causes severe oxidative stress; (3) the rate of ATP generation based on adipose tissue-derived fatty acids is slower than that using blood glucose as fuel. Thus, in periods of extended continuous and rapid neuronal firing, fatty acid oxidation cannot guarantee rapid ATP generation in neurons. We conjecture that the disadvantages connected with using fatty acids as fuel have created evolutionary pressure on lowering the expression of the β-oxidation enzyme equipment in brain mitochondria to avoid extensive fatty acid oxidation and to favor glucose oxidation in brain.
Figures
Similar articles
-
Brain energy metabolism spurns fatty acids as fuel due to their inherent mitotoxicity and potential capacity to unleash neurodegeneration.Neurochem Int. 2017 Oct;109:68-77. doi: 10.1016/j.neuint.2017.03.018. Epub 2017 Mar 30. Neurochem Int. 2017. PMID: 28366720 Review.
-
How the brain fights fatty acids' toxicity.Neurochem Int. 2021 Sep;148:105050. doi: 10.1016/j.neuint.2021.105050. Epub 2021 May 1. Neurochem Int. 2021. PMID: 33945834 Review.
-
Glucose metabolism and metabolic flexibility in blood platelets.J Thromb Haemost. 2018 Nov;16(11):2300-2314. doi: 10.1111/jth.14274. Epub 2018 Sep 27. J Thromb Haemost. 2018. PMID: 30151891
-
GSEA of mouse and human mitochondriomes reveals fatty acid oxidation in astrocytes.Glia. 2018 Aug;66(8):1724-1735. doi: 10.1002/glia.23330. Epub 2018 Mar 25. Glia. 2018. PMID: 29575211
-
Fatty acids in energy metabolism of the central nervous system.Biomed Res Int. 2014;2014:472459. doi: 10.1155/2014/472459. Epub 2014 May 4. Biomed Res Int. 2014. PMID: 24883315 Free PMC article. Review.
Cited by
-
In the Brain, It Is Not All about Sugar.NeuroSci. 2024 Jun 19;5(2):209-221. doi: 10.3390/neurosci5020016. eCollection 2024 Jun. NeuroSci. 2024. PMID: 39483499 Free PMC article. Review.
-
The Therapeutic Potential of Glucagon-like Peptide 1 Receptor Agonists in Traumatic Brain Injury.Pharmaceuticals (Basel). 2024 Oct 1;17(10):1313. doi: 10.3390/ph17101313. Pharmaceuticals (Basel). 2024. PMID: 39458954 Free PMC article. Review.
-
Brain Metabolism in Health and Neurodegeneration: The Interplay Among Neurons and Astrocytes.Cells. 2024 Oct 17;13(20):1714. doi: 10.3390/cells13201714. Cells. 2024. PMID: 39451233 Free PMC article. Review.
-
LDL Exposure Disrupts Mitochondrial Function and Dynamics in a Hippocampal Neuronal Cell Line.Mol Neurobiol. 2024 Sep 20. doi: 10.1007/s12035-024-04476-y. Online ahead of print. Mol Neurobiol. 2024. PMID: 39302616
-
Effect of ketone monoester supplementation on elite operators' mountaineering training.Front Physiol. 2024 Sep 3;15:1411421. doi: 10.3389/fphys.2024.1411421. eCollection 2024. Front Physiol. 2024. PMID: 39290617 Free PMC article.
References
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
-
- Clarke DD, Sokoloff L.Circulation and energy metabolism of the brainIn: Siegel GJ, Agranoff BW, Albers RW, Molinoff PB, (eds). Basic Neurochemistry Raven Press: New York; 1994645–680.
-
- Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Res Bull. 2008;76:329–343. - PubMed
-
- Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

