Increased matrix metalloproteinase (MMPs) levels do not predict disease severity or progression in emphysema
- PMID: 23441181
- PMCID: PMC3575373
- DOI: 10.1371/journal.pone.0056352
Increased matrix metalloproteinase (MMPs) levels do not predict disease severity or progression in emphysema
Abstract
Rationale: Though matrix metalloproteinases (MMPs) are critical in the pathogenesis of COPD, their utility as a disease biomarker remains uncertain. This study aimed to determine whether bronchoalveolar lavage (BALF) or plasma MMP measurements correlated with disease severity or functional decline in emphysema.
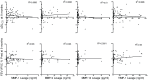
Methods: Enzyme-linked immunosorbent assay and luminex assays measured MMP-1, -9, -12 and tissue inhibitor of matrix metalloproteinase-1 in the BALF and plasma of non-smokers, smokers with normal lung function and moderate-to-severe emphysema subjects. In the cohort of 101 emphysema subjects correlative analyses were done to determine if MMP or TIMP-1 levels were associated with key disease parameters or change in lung function over an 18-month time period.
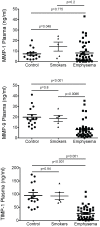
Main results: Compared to non-smoking controls, MMP and TIMP-1 BALF levels were significantly elevated in the emphysema cohort. Though MMP-1 was elevated in both the normal smoker and emphysema groups, collagenase activity was only increased in the emphysema subjects. In contrast to BALF, plasma MMP-9 and TIMP-1 levels were actually decreased in the emphysema cohort compared to the control groups. Both in the BALF and plasma, MMP and TIMP-1 measurements in the emphysema subjects did not correlate with important disease parameters and were not predictive of subsequent functional decline.
Conclusions: MMPs are altered in the BALF and plasma of emphysema; however, the changes in MMPs correlate poorly with parameters of disease intensity or progression. Though MMPs are pivotal in the pathogenesis of COPD, these findings suggest that measuring MMPs will have limited utility as a prognostic marker in this disease.
Conflict of interest statement
Figures





Similar articles
-
Distinct emphysema subtypes defined by quantitative CT analysis are associated with specific pulmonary matrix metalloproteinases.Respir Res. 2016 Jul 26;17(1):92. doi: 10.1186/s12931-016-0402-z. Respir Res. 2016. PMID: 27460105 Free PMC article.
-
SIRT1 redresses the imbalance of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-9 in the development of mouse emphysema and human COPD.Am J Physiol Lung Cell Mol Physiol. 2013 Nov 1;305(9):L615-24. doi: 10.1152/ajplung.00249.2012. Epub 2013 Sep 13. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 24039251 Free PMC article.
-
Comparative Study of Cytokine Levels in Different Respiratory Samples in Mild-to-Moderate AECOPD Patients.Lung. 2019 Oct;197(5):565-572. doi: 10.1007/s00408-019-00263-y. Epub 2019 Aug 26. Lung. 2019. PMID: 31451927
-
Series "matrix metalloproteinases in lung health and disease": Matrix metalloproteinases in COPD.Eur Respir J. 2012 Jan;39(1):197-209. doi: 10.1183/09031936.00121611. Epub 2011 Sep 15. Eur Respir J. 2012. PMID: 21920892 Review.
-
Matrix Metalloproteinases in Chronic Obstructive Pulmonary Disease.Int J Mol Sci. 2023 Feb 14;24(4):3786. doi: 10.3390/ijms24043786. Int J Mol Sci. 2023. PMID: 36835197 Free PMC article. Review.
Cited by
-
Biomarkers of World Trade Center Particulate Matter Exposure: Physiology of Distal Airway and Blood Biomarkers that Predict FEV₁ Decline.Semin Respir Crit Care Med. 2015 Jun;36(3):323-33. doi: 10.1055/s-0035-1547349. Epub 2015 May 29. Semin Respir Crit Care Med. 2015. PMID: 26024341 Free PMC article. Review.
-
Early elevation of serum MMP-3 and MMP-12 predicts protection from World Trade Center-lung injury in New York City Firefighters: a nested case-control study.PLoS One. 2013 Oct 16;8(10):e76099. doi: 10.1371/journal.pone.0076099. eCollection 2013. PLoS One. 2013. PMID: 24146820 Free PMC article.
-
Anti-CELA1 antibody KF4 prevents emphysema by inhibiting stretch-mediated remodeling.JCI Insight. 2024 Jan 9;9(1):e169189. doi: 10.1172/jci.insight.169189. JCI Insight. 2024. PMID: 38193533 Free PMC article.
-
Distinct emphysema subtypes defined by quantitative CT analysis are associated with specific pulmonary matrix metalloproteinases.Respir Res. 2016 Jul 26;17(1):92. doi: 10.1186/s12931-016-0402-z. Respir Res. 2016. PMID: 27460105 Free PMC article.
-
Impact of Matrix Metalloproteinase 9 on COPD Development in Polish Patients: Genetic Polymorphism, Protein Level, and Their Relationship with Lung Function.Biomed Res Int. 2018 Dec 10;2018:6417415. doi: 10.1155/2018/6417415. eCollection 2018. Biomed Res Int. 2018. PMID: 30643813 Free PMC article.
References
-
- Shiomi T, Okada Y, Foronjy R, Schiltz J, Jaenish R, et al. (2001) Emphysematous Changes Are Caused By Degradation Of Type III Collagen In Transgenic Mice Expressing MMP-1. Exp Lung Res. - PubMed
-
- Hu J, Van den Steen PE, Sang QX, Opdenakker G (2007) Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov 6: 480–498. - PubMed
-
- Finlay GA, O’Driscoll L, Russell KJ, D’Arcy EM, Masterson JB, et al. (1997) Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. American Journal Respiratory Critical Care Medicine 156: 240–247. - PubMed
-
- Ohnishi K, Takagi M, Kurokawa Y, Satomi S, Konttinen YT (1998) Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest 78: 1077–1087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

