Integrated microdevice of reverse transcription-polymerase chain reaction with colorimetric immunochromatographic detection for rapid gene expression analysis of influenza A H1N1 virus
- PMID: 22265877
- PMCID: PMC7126693
- DOI: 10.1016/j.bios.2011.12.024
Integrated microdevice of reverse transcription-polymerase chain reaction with colorimetric immunochromatographic detection for rapid gene expression analysis of influenza A H1N1 virus
Abstract
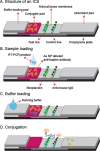
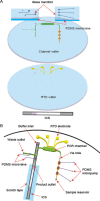
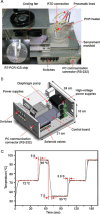
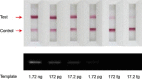
An integrated microdevice of a reverse transcription-polymerase chain reaction (RT-PCR) reactor and an immunochromatographic strip was constructed for colorimetric detection of gene expression of influenza A virus subtype H1N1. An RT-PCR cocktail, which included Texas Red-labeled primers, dNTP including biotin-labeled dUTP, and RNA templates of influenza A H1N1 virus, was filled in the PCR chamber through the micropump, and the RT-PCR was performed to amplify the target H1 gene (102 bp). The resultant amplicons bearing biotin moieties and Texas Red haptens were subsequently eluted to the immunochromatographic strip, in which they were first conjugated with the gold nanoparticle labeled anti-hapten antibody in the conjugation pad, and then captured on the streptavidin coated test line through the biotin-streptavidin interaction. By observing a violet color in the test line which was derived from the gold nanoparticle, we confirmed the H1N1 target virus. The entire process on the integrated microdevice consisting of a micropump, a 2 μL PCR chamber, and an immunochromatographic strip was carried out on the portable genetic analyzer within 2.5h, enabling on-site colorimetric pathogen identification with detection sensitivity of 14.1 pg RNA templates.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Combination of multiplex reverse-transcription loop-mediated isothermal amplification with an immunochromatographic strip for subtyping influenza A virus.Anal Chim Acta. 2015 Jan 1;853:541-547. doi: 10.1016/j.aca.2014.10.020. Epub 2014 Oct 16. Anal Chim Acta. 2015. PMID: 25467501 Free PMC article.
-
A packaged paper fluidic-based microdevice for detecting gene expression of influenza A virus.Biosens Bioelectron. 2014 Nov 15;61:485-90. doi: 10.1016/j.bios.2014.06.006. Epub 2014 Jun 8. Biosens Bioelectron. 2014. PMID: 24949821
-
Integration of reverse transcriptase loop-mediated isothermal amplification with an immunochromatographic strip on a centrifugal microdevice for influenza A virus identification.Lab Chip. 2015 Feb 7;15(3):718-25. doi: 10.1039/c4lc01033g. Lab Chip. 2015. PMID: 25426967
-
[Colorimetric detection of human influenza A H1N1 virus by reverse transcription loop mediated isothermal amplification].Bing Du Xue Bao. 2010 Mar;26(2):81-7. Bing Du Xue Bao. 2010. PMID: 20480635 Chinese.
-
Integrated centrifugal reverse transcriptase loop-mediated isothermal amplification microdevice for influenza A virus detection.Biosens Bioelectron. 2015 Jun 15;68:218-224. doi: 10.1016/j.bios.2014.12.043. Epub 2014 Dec 23. Biosens Bioelectron. 2015. PMID: 25569879 Free PMC article.
Cited by
-
A simple and novel way of maintaining constant wall temperature in microdevices.Sci Rep. 2016 Jan 22;6:18230. doi: 10.1038/srep18230. Sci Rep. 2016. PMID: 26795753 Free PMC article.
-
Combination of multiplex reverse-transcription loop-mediated isothermal amplification with an immunochromatographic strip for subtyping influenza A virus.Anal Chim Acta. 2015 Jan 1;853:541-547. doi: 10.1016/j.aca.2014.10.020. Epub 2014 Oct 16. Anal Chim Acta. 2015. PMID: 25467501 Free PMC article.
-
Towards Multiplex Molecular Diagnosis-A Review of Microfluidic Genomics Technologies.Micromachines (Basel). 2017 Aug 30;8(9):266. doi: 10.3390/mi8090266. Micromachines (Basel). 2017. PMID: 30400456 Free PMC article. Review.
-
Combined use of vancomycin-modified Ag-coated magnetic nanoparticles and secondary enhanced nanoparticles for rapid surface-enhanced Raman scattering detection of bacteria.Int J Nanomedicine. 2018 Feb 27;13:1159-1178. doi: 10.2147/IJN.S150336. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29520142 Free PMC article.
-
Enhanced DNA sensing via catalytic aggregation of gold nanoparticles.Biosens Bioelectron. 2013 Dec 15;50:382-6. doi: 10.1016/j.bios.2013.06.063. Epub 2013 Jul 11. Biosens Bioelectron. 2013. PMID: 23891867 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

