Sustained axon regeneration induced by co-deletion of PTEN and SOCS3
- PMID: 22056987
- PMCID: PMC3240702
- DOI: 10.1038/nature10594
Sustained axon regeneration induced by co-deletion of PTEN and SOCS3
Abstract
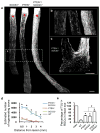
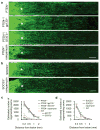
A formidable challenge in neural repair in the adult central nervous system (CNS) is the long distances that regenerating axons often need to travel in order to reconnect with their targets. Thus, a sustained capacity for axon regeneration is critical for achieving functional restoration. Although deletion of either phosphatase and tensin homologue (PTEN), a negative regulator of mammalian target of rapamycin (mTOR), or suppressor of cytokine signalling 3 (SOCS3), a negative regulator of Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway, in adult retinal ganglion cells (RGCs) individually promoted significant optic nerve regeneration, such regrowth tapered off around 2 weeks after the crush injury. Here we show that, remarkably, simultaneous deletion of both PTEN and SOCS3 enables robust and sustained axon regeneration. We further show that PTEN and SOCS3 regulate two independent pathways that act synergistically to promote enhanced axon regeneration. Gene expression analyses suggest that double deletion not only results in the induction of many growth-related genes, but also allows RGCs to maintain the expression of a repertoire of genes at the physiological level after injury. Our results reveal concurrent activation of mTOR and STAT3 pathways as key for sustaining long-distance axon regeneration in adult CNS, a crucial step towards functional recovery.
Conflict of interest statement
Figures


Similar articles
-
Impact of PTEN/SOCS3 deletion on amelioration of dendritic shrinkage of retinal ganglion cells after optic nerve injury.Exp Eye Res. 2020 Mar;192:107938. doi: 10.1016/j.exer.2020.107938. Epub 2020 Jan 21. Exp Eye Res. 2020. PMID: 31972211
-
Modest enhancement of sensory axon regeneration in the sciatic nerve with conditional co-deletion of PTEN and SOCS3 in the dorsal root ganglia of adult mice.Exp Neurol. 2018 May;303:120-133. doi: 10.1016/j.expneurol.2018.02.012. Epub 2018 Feb 16. Exp Neurol. 2018. PMID: 29458059 Free PMC article.
-
Three-dimensional evaluation of retinal ganglion cell axon regeneration and pathfinding in whole mouse tissue after injury.Exp Neurol. 2013 Sep;247:653-62. doi: 10.1016/j.expneurol.2013.03.001. Epub 2013 Mar 16. Exp Neurol. 2013. PMID: 23510761 Free PMC article.
-
Lab review: Molecular dissection of the signal transduction pathways associated with PTEN deletion-induced optic nerve regeneration.Restor Neurol Neurosci. 2019;37(6):545-552. doi: 10.3233/RNN-190949. Restor Neurol Neurosci. 2019. PMID: 31839616 Free PMC article. Review.
-
Lost in the jungle: new hurdles for optic nerve axon regeneration.Trends Neurosci. 2014 Jul;37(7):381-7. doi: 10.1016/j.tins.2014.05.002. Epub 2014 May 26. Trends Neurosci. 2014. PMID: 24874558 Review.
Cited by
-
Signaling pathways that regulate axon regeneration.Neurosci Bull. 2013 Aug;29(4):411-20. doi: 10.1007/s12264-013-1357-4. Epub 2013 Jul 11. Neurosci Bull. 2013. PMID: 23846598 Free PMC article. Review.
-
Emergent properties of neural repair: elemental biology to therapeutic concepts.Ann Neurol. 2016 Jun;79(6):895-906. doi: 10.1002/ana.24653. Epub 2016 Apr 21. Ann Neurol. 2016. PMID: 27043816 Free PMC article. Review.
-
Bridging the gap of axonal regeneration in the central nervous system: A state of the art review on central axonal regeneration.Front Neurosci. 2022 Nov 9;16:1003145. doi: 10.3389/fnins.2022.1003145. eCollection 2022. Front Neurosci. 2022. PMID: 36440273 Free PMC article. Review.
-
Chapter 5 - Restoring Vision to the Blind: Endogenous Regeneration.Transl Vis Sci Technol. 2014 Dec 30;3(7):7. doi: 10.1167/tvst.3.7.7. eCollection 2014 Dec. Transl Vis Sci Technol. 2014. PMID: 25653891 Free PMC article. No abstract available.
-
Neuroinflammation triggered by β-glucan/dectin-1 signaling enables CNS axon regeneration.Proc Natl Acad Sci U S A. 2015 Feb 24;112(8):2581-6. doi: 10.1073/pnas.1423221112. Epub 2015 Feb 9. Proc Natl Acad Sci U S A. 2015. PMID: 25675510 Free PMC article.
References
-
- Fawcett J. Molecular control of brain plasticity and repair. Prog Brain Res. 2009;175:501–509. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

