Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion
- PMID: 21984579
- PMCID: PMC3198081
- DOI: 10.2337/db11-0132
Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion
Abstract
Objective: The role of uncoupling protein 2 (UCP2) in pancreatic β-cells is highly debated, partly because of the broad tissue distribution of UCP2 and thus limitations of whole-body UCP2 knockout mouse models. To investigate the function of UCP2 in the β-cell, β-cell-specific UCP2 knockout mice (UCP2BKO) were generated and characterized.
Research design and methods: UCP2BKO mice were generated by crossing loxUCP2 mice with mice expressing rat insulin promoter-driven Cre recombinase. Several in vitro and in vivo parameters were measured, including respiration rate, mitochondrial membrane potential, islet ATP content, reactive oxygen species (ROS) levels, glucose-stimulated insulin secretion (GSIS), glucagon secretion, glucose and insulin tolerance, and plasma hormone levels.
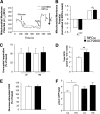
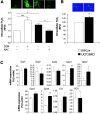
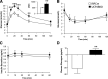
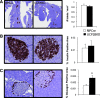
Results: UCP2BKO β-cells displayed mildly increased glucose-induced mitochondrial membrane hyperpolarization but unchanged rates of uncoupled respiration and islet ATP content. UCP2BKO islets had elevated intracellular ROS levels that associated with enhanced GSIS. Surprisingly, UCP2BKO mice were glucose-intolerant, showing greater α-cell area, higher islet glucagon content, and aberrant ROS-dependent glucagon secretion under high glucose conditions.
Conclusions: Using a novel β-cell-specific UCP2KO mouse model, we have shed light on UCP2 function in primary β-cells. UCP2 does not behave as a classical metabolic uncoupler in the β-cell, but has a more prominent role in the regulation of intracellular ROS levels that contribute to GSIS amplification. In addition, β-cell UCP2 contributes to the regulation of intraislet ROS signals that mediate changes in α-cell morphology and glucagon secretion.
Figures



Similar articles
-
Uncoupling protein 2 regulates reactive oxygen species formation in islets and influences susceptibility to diabetogenic action of streptozotocin.J Endocrinol. 2009 Oct;203(1):33-43. doi: 10.1677/JOE-09-0117. Epub 2009 Jul 27. J Endocrinol. 2009. PMID: 19635759
-
UCP2 regulates the glucagon response to fasting and starvation.Diabetes. 2013 May;62(5):1623-33. doi: 10.2337/db12-0981. Epub 2013 Feb 22. Diabetes. 2013. PMID: 23434936 Free PMC article.
-
Increasing uncoupling protein-2 in pancreatic beta cells does not alter glucose-induced insulin secretion but decreases production of reactive oxygen species.Diabetologia. 2007 Jan;50(1):84-93. doi: 10.1007/s00125-006-0499-6. Epub 2006 Nov 28. Diabetologia. 2007. PMID: 17131143
-
Reactive oxygen species and uncoupling protein 2 in pancreatic β-cell function.Diabetes Obes Metab. 2010 Oct;12 Suppl 2:141-8. doi: 10.1111/j.1463-1326.2010.01269.x. Diabetes Obes Metab. 2010. PMID: 21029311 Review.
-
Mitochondrial uncoupling protein 2 in pancreatic β-cells.Diabetes Obes Metab. 2010 Oct;12 Suppl 2:134-40. doi: 10.1111/j.1463-1326.2010.01264.x. Diabetes Obes Metab. 2010. PMID: 21029310 Review.
Cited by
-
Ferroptosis and iron metabolism in diabetes: Pathogenesis, associated complications, and therapeutic implications.Front Endocrinol (Lausanne). 2024 Aug 30;15:1447148. doi: 10.3389/fendo.2024.1447148. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39279996 Free PMC article. Review.
-
The Proton Leak of the Inner Mitochondrial Membrane Is Enlarged in Freshly Isolated Pancreatic Islets.Biomedicines. 2024 Aug 2;12(8):1747. doi: 10.3390/biomedicines12081747. Biomedicines. 2024. PMID: 39200212 Free PMC article.
-
The Regulation of Metabolic Homeostasis by Incretins and the Metabolic Hormones Produced by Pancreatic Islets.Diabetes Metab Syndr Obes. 2024 Jun 13;17:2419-2456. doi: 10.2147/DMSO.S415934. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 38894706 Free PMC article. Review.
-
FOXA1 and FOXA2: the regulatory mechanisms and therapeutic implications in cancer.Cell Death Discov. 2024 Apr 11;10(1):172. doi: 10.1038/s41420-024-01936-1. Cell Death Discov. 2024. PMID: 38605023 Free PMC article. Review.
-
Mitochondrial bioenergetics, metabolism, and beyond in pancreatic β-cells and diabetes.Front Mol Biosci. 2024 Feb 9;11:1354199. doi: 10.3389/fmolb.2024.1354199. eCollection 2024. Front Mol Biosci. 2024. PMID: 38404962 Free PMC article. Review.
References
-
- Fleury C, Neverova M, Collins S, et al. . Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet 1997;15:269–272 - PubMed
-
- Echtay KS. Mitochondrial uncoupling proteins—what is their physiological role? Free Radic Biol Med 2007;43:1351–1371 - PubMed
-
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab 2005;2:85–93 - PubMed
-
- Echtay KS, Roussel D, St-Pierre J, et al. . Superoxide activates mitochondrial uncoupling proteins. Nature 2002;415:96–99 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

