Kallikrein-related peptidase 14 acts on proteinase-activated receptor 2 to induce signaling pathway in colon cancer cells
- PMID: 21907696
- PMCID: PMC3204030
- DOI: 10.1016/j.ajpath.2011.07.016
Kallikrein-related peptidase 14 acts on proteinase-activated receptor 2 to induce signaling pathway in colon cancer cells
Abstract
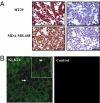
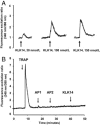
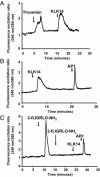
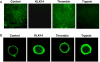
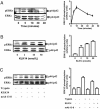
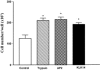
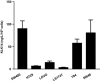
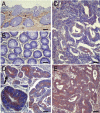
Serine proteinases participate in tumor growth and invasion by cleaving and activating proteinase-activated receptors (PARs). Recent studies have implicated PAR-1 and PAR-4 (activated by thrombin) and PAR-2 (activated by trypsin but not by thrombin) in human colon cancer growth. The endogenous activators of PARs in colon tumors, however, are still unknown. We hypothesize that the kallikrein-related peptidase (KLK) family member KLK14, a known tumor biomarker, is produced by colonic tumors and signals to human colon cancer cells by activating PARs. We found that i) KLK14 mRNA was present in 16 human colon cancer cell lines, ii) KLK14 protein was expressed and secreted in colon cancer cell lines, and iii) KLK14 (0.1 μmol/L) induced increases in intracellular calcium in HT29, a human colon cancer-derived cell line. KLK14-induced calcium flux was associated with internalization of KLK14-mediated activation of PAR-2. Furthermore, KLK14 induced significant extracellular signal-regulated kinases 1 and 2 (ERK1/2) phosphorylation and HT29 cell proliferation, presumably by activating PAR-2. A PAR-2 cleavage and activation-blocking antibody dramatically reduced KLK14-induced ERK1/2 signaling. Finally, ectopic expression of KLK14 in human colon adenocarcinomas and its absence in normal epithelia was demonstrated by IHC analysis. These results demonstrate, for the first time, the aberrant expression of KLK14 in colon cancer and its involvement in PAR-2 receptor signaling. Thus, KLK14 and its receptor, PAR-2, may represent therapeutic targets for colon tumorigenesis.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Kallikrein-related peptidase signaling in colon carcinoma cells: targeting proteinase-activated receptors.Biol Chem. 2012 Apr;393(5):413-20. doi: 10.1515/bc-2011-231. Biol Chem. 2012. PMID: 22505523
-
Kallikrein-related peptidase 4: a new activator of the aberrantly expressed protease-activated receptor 1 in colon cancer cells.Am J Pathol. 2010 Mar;176(3):1452-61. doi: 10.2353/ajpath.2010.090523. Epub 2010 Jan 7. Am J Pathol. 2010. PMID: 20056842 Free PMC article.
-
Proteinase-activated receptors (PARs): differential signalling by kallikrein-related peptidases KLK8 and KLK14.Biol Chem. 2012 Apr;393(5):421-7. doi: 10.1515/hsz-2011-0251. Biol Chem. 2012. PMID: 22505524
-
Kallikreins and proteinase-mediated signaling: proteinase-activated receptors (PARs) and the pathophysiology of inflammatory diseases and cancer.Biol Chem. 2008 Jun;389(6):643-51. doi: 10.1515/BC.2008.077. Biol Chem. 2008. PMID: 18627296 Review.
-
Prostatic trypsin-like kallikrein-related peptidases (KLKs) and other prostate-expressed tryptic proteinases as regulators of signalling via proteinase-activated receptors (PARs).Biol Chem. 2008 Jun;389(6):653-68. doi: 10.1515/BC.2008.078. Biol Chem. 2008. PMID: 18627286 Review.
Cited by
-
Upregulation of KLK8 Predicts Poor Prognosis in Pancreatic Cancer.Front Oncol. 2021 Jul 30;11:624837. doi: 10.3389/fonc.2021.624837. eCollection 2021. Front Oncol. 2021. PMID: 34395235 Free PMC article.
-
Proteinase-activated receptors (PARs) - focus on receptor-receptor-interactions and their physiological and pathophysiological impact.Cell Commun Signal. 2013 Nov 11;11:86. doi: 10.1186/1478-811X-11-86. Cell Commun Signal. 2013. PMID: 24215724 Free PMC article. Review.
-
Role of Serine Proteases at the Tumor-Stroma Interface.Front Immunol. 2022 Feb 11;13:832418. doi: 10.3389/fimmu.2022.832418. eCollection 2022. Front Immunol. 2022. PMID: 35222418 Free PMC article. Review.
-
Protease-activated receptor-2 accelerates intestinal tumor formation through activation of nuclear factor-κB signaling and tumor angiogenesis in ApcMin/+ mice.Cancer Sci. 2020 Apr;111(4):1193-1202. doi: 10.1111/cas.14335. Epub 2020 Feb 24. Cancer Sci. 2020. PMID: 31997435 Free PMC article.
-
Induction of complement C3a receptor responses by kallikrein-related peptidase 14.J Immunol. 2013 Oct 1;191(7):3858-66. doi: 10.4049/jimmunol.1202999. Epub 2013 Sep 6. J Immunol. 2013. PMID: 24014879 Free PMC article.
References
-
- Mook O.R., Frederiks W.M., Van Noorden C.J. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta. 2004;1705:69–89. - PubMed
-
- Lopez-Otin C., Matrisian L.M. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. - PubMed
-
- Dery O., Corvera C.U., Steinhoff M., Bunnett N.W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol. 1998;274:C1429–C1452. - PubMed
-
- Macfarlane S.R., Seatter M.J., Kanke T., Hunter G.D., Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. - PubMed
-
- Ossovskaya V.S., Bunnett N.W. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

