Integration of auditory and somatosensory error signals in the neural control of speech movements
- PMID: 21562187
- PMCID: PMC3154803
- DOI: 10.1152/jn.00638.2010
Integration of auditory and somatosensory error signals in the neural control of speech movements
Abstract
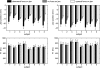
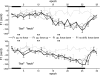
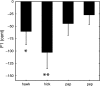
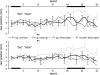
We investigated auditory and somatosensory feedback contributions to the neural control of speech. In task I, sensorimotor adaptation was studied by perturbing one of these sensory modalities or both modalities simultaneously. The first formant (F1) frequency in the auditory feedback was shifted up by a real-time processor and/or the extent of jaw opening was increased or decreased with a force field applied by a robotic device. All eight subjects lowered F1 to compensate for the up-shifted F1 in the feedback signal regardless of whether or not the jaw was perturbed. Adaptive changes in subjects' acoustic output resulted from adjustments in articulatory movements of the jaw or tongue. Adaptation in jaw opening extent in response to the mechanical perturbation occurred only when no auditory feedback perturbation was applied or when the direction of adaptation to the force was compatible with the direction of adaptation to a simultaneous acoustic perturbation. In tasks II and III, subjects' auditory and somatosensory precision and accuracy were estimated. Correlation analyses showed that the relationships 1) between F1 adaptation extent and auditory acuity for F1 and 2) between jaw position adaptation extent and somatosensory acuity for jaw position were weak and statistically not significant. Taken together, the combined findings from this work suggest that, in speech production, sensorimotor adaptation updates the underlying control mechanisms in such a way that the planning of vowel-related articulatory movements takes into account a complex integration of error signals from previous trials but likely with a dominant role for the auditory modality.
Figures

Similar articles
-
Sensorimotor adaptation to feedback perturbations of vowel acoustics and its relation to perception.J Acoust Soc Am. 2007 Oct;122(4):2306-19. doi: 10.1121/1.2773966. J Acoust Soc Am. 2007. PMID: 17902866
-
Adaptation strategies in perturbed /s/.Clin Linguist Phon. 2011 Aug;25(8):705-24. doi: 10.3109/02699206.2011.553699. Epub 2011 Mar 18. Clin Linguist Phon. 2011. PMID: 21417737
-
Somatosensory basis of speech production.Nature. 2003 Jun 19;423(6942):866-9. doi: 10.1038/nature01710. Nature. 2003. PMID: 12815431
-
Jaw sensorimotor control in healthy adults and effects of ageing.J Oral Rehabil. 2018 Jan;45(1):50-80. doi: 10.1111/joor.12554. Epub 2017 Oct 6. J Oral Rehabil. 2018. PMID: 28853161 Review.
-
Internal models in sensorimotor integration: perspectives from adaptive control theory.J Neural Eng. 2005 Sep;2(3):S147-63. doi: 10.1088/1741-2560/2/3/S01. Epub 2005 Aug 31. J Neural Eng. 2005. PMID: 16135881 Free PMC article. Review.
Cited by
-
Divided Attention Has Limited Effects on Speech Sensorimotor Control.J Speech Lang Hear Res. 2024 Nov 7;67(11):4358-4368. doi: 10.1044/2024_JSLHR-24-00098. Epub 2024 Oct 17. J Speech Lang Hear Res. 2024. PMID: 39418590
-
Speech sensorimotor relationships in francophone preschoolers and adults: Adaptation to real-time auditory feedback perturbations.PLoS One. 2024 Aug 22;19(8):e0306246. doi: 10.1371/journal.pone.0306246. eCollection 2024. PLoS One. 2024. PMID: 39172970 Free PMC article.
-
Theta-burst stimulation over primary somatosensory cortex modulates tactile acuity of tongue.bioRxiv [Preprint]. 2024 Jun 18:2024.06.17.599457. doi: 10.1101/2024.06.17.599457. bioRxiv. 2024. PMID: 38948808 Free PMC article. Preprint.
-
Quick speech motor correction in the absence of auditory feedback.Front Hum Neurosci. 2024 Jun 6;18:1399316. doi: 10.3389/fnhum.2024.1399316. eCollection 2024. Front Hum Neurosci. 2024. PMID: 38903407 Free PMC article.
-
Neurophysiological evidence of sensory prediction errors driving speech sensorimotor adaptation.bioRxiv [Preprint]. 2024 Sep 17:2023.10.22.563504. doi: 10.1101/2023.10.22.563504. bioRxiv. 2024. PMID: 37961099 Free PMC article. Preprint.
References
-
- Abbs JH, Gracco VL. Control of complex motor gestures: orofacial muscle responses to load perturbations of lip during speech. J Neurophysiol 51: 705–723, 1984 - PubMed
-
- Battaglia PW, Jacobs RA, Aslin RN. Bayesian integration of visual and auditory signals for spatial localization. J Opt Soc Am A Opt Image Sci Vis 20: 1391–1397, 2003 - PubMed
-
- Baum SR, McFarland DH. The development of speech adaptation to an artificial palate. J Acoust Soc Am 102: 2353–2359, 1997 - PubMed
-
- Burnett TA, Freedland MB, Larson CR, Hain TC. Voice F0 responses to manipulations in pitch feedback. J Acoust Soc Am 103: 3153–3161, 1998 - PubMed
-
- Cohen HB. Some critical factors in prism-adaptation. Am J Psychol 79: 285–290, 1966 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

