Recombinant AAV-directed gene therapy for type I glycogen storage diseases
- PMID: 21504389
- PMCID: PMC3126888
- DOI: 10.1517/14712598.2011.578067
Recombinant AAV-directed gene therapy for type I glycogen storage diseases
Abstract
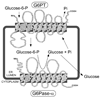
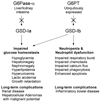
Introduction: Glycogen storage disease (GSD) type Ia and Ib are disorders of impaired glucose homeostasis affecting the liver and kidney. GSD-Ib also affects neutrophils. Current dietary therapies cannot prevent long-term complications. In animal studies, recombinant adeno-associated virus (rAAV) vector-mediated gene therapy can correct or minimize multiple aspects of the disorders, offering hope for human gene therapy.
Areas covered: A summary of recent progress in rAAV-mediated gene therapy for GSD-I; strategies to improve rAAV-mediated gene delivery, transduction efficiency and immune avoidance; and vector refinements that improve expression.
Expert opinion: rAAV-mediated gene delivery to the liver can restore glucose homeostasis in preclinical models of GSD-I, but some long-term complications of the liver and kidney remain. Gene therapy for GSD-Ib is less advanced than for GSD-Ia and only transient correction of myeloid dysfunction has been achieved. A question remains as to whether a single rAAV vector can meet the expression efficiency and tropism required to treat all aspects of GSD-I, or if a multi-pronged approach is needed. An understanding of the strengths and weaknesses of rAAV vectors in the context of strategies to achieve efficient transduction of the liver, kidney and hematopoietic stem cells is required for treating GSD-I.
Figures
Similar articles
-
Gene therapy using a novel G6PC-S298C variant enhances the long-term efficacy for treating glycogen storage disease type Ia.Biochem Biophys Res Commun. 2020 Jun 30;527(3):824-830. doi: 10.1016/j.bbrc.2020.04.124. Epub 2020 May 16. Biochem Biophys Res Commun. 2020. PMID: 32430177 Free PMC article.
-
Molecular biology and gene therapy for glycogen storage disease type Ib.J Inherit Metab Dis. 2018 Nov;41(6):1007-1014. doi: 10.1007/s10545-018-0180-5. Epub 2018 Apr 16. J Inherit Metab Dis. 2018. PMID: 29663270 Review.
-
Glycogen storage disease type Ia mice with less than 2% of normal hepatic glucose-6-phosphatase-α activity restored are at risk of developing hepatic tumors.Mol Genet Metab. 2017 Mar;120(3):229-234. doi: 10.1016/j.ymgme.2017.01.003. Epub 2017 Jan 10. Mol Genet Metab. 2017. PMID: 28096054 Free PMC article.
-
An evolutionary approach to optimizing glucose-6-phosphatase-α enzymatic activity for gene therapy of glycogen storage disease type Ia.J Inherit Metab Dis. 2019 May;42(3):470-479. doi: 10.1002/jimd.12069. Epub 2019 Feb 22. J Inherit Metab Dis. 2019. PMID: 30714174 Free PMC article.
-
Emerging roles of autophagy in hepatic tumorigenesis and therapeutic strategies in glycogen storage disease type Ia: A review.J Inherit Metab Dis. 2021 Jan;44(1):118-128. doi: 10.1002/jimd.12267. Epub 2020 Jul 2. J Inherit Metab Dis. 2021. PMID: 32474930 Review.
Cited by
-
Gene therapy and genome editing for type I glycogen storage diseases.Front Mol Med. 2023 Mar 31;3:1167091. doi: 10.3389/fmmed.2023.1167091. eCollection 2023. Front Mol Med. 2023. PMID: 39086673 Free PMC article. Review.
-
Genome-wide RNAi screening identifies host restriction factors critical for in vivo AAV transduction.Proc Natl Acad Sci U S A. 2015 Sep 8;112(36):11276-81. doi: 10.1073/pnas.1503607112. Epub 2015 Aug 24. Proc Natl Acad Sci U S A. 2015. PMID: 26305933 Free PMC article.
-
Type I glycogen storage diseases: disorders of the glucose-6-phosphatase/glucose-6-phosphate transporter complexes.J Inherit Metab Dis. 2015 May;38(3):511-9. doi: 10.1007/s10545-014-9772-x. Epub 2014 Oct 7. J Inherit Metab Dis. 2015. PMID: 25288127 Review.
-
Prevention of hepatocellular adenoma and correction of metabolic abnormalities in murine glycogen storage disease type Ia by gene therapy.Hepatology. 2012 Nov;56(5):1719-29. doi: 10.1002/hep.25717. Epub 2012 Aug 27. Hepatology. 2012. PMID: 22422504 Free PMC article.
-
From COVID-19 to Cancer mRNA Vaccines: Moving From Bench to Clinic in the Vaccine Landscape.Front Immunol. 2021 Jul 7;12:679344. doi: 10.3389/fimmu.2021.679344. eCollection 2021. Front Immunol. 2021. PMID: 34305909 Free PMC article. Review.
References
-
- Chou JY, Matern D, Mansfield BC, et al. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr Mol Med. 2002;2:121–143. - PubMed
-
- Lei K-J, Shelly LL, Pan C-J, et al. Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type 1a. Science. 1993;262:580–583. - PubMed
-
- Pan C-J, Lei K-J, Annabi B, et al. Transmembrane topology of glucose-6-phosphatase. J Biol Chem. 1998;273:6144–6148. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
