A disposable, self-contained PCR chip
- PMID: 19190797
- PMCID: PMC2676225
- DOI: 10.1039/b807915c
A disposable, self-contained PCR chip
Abstract
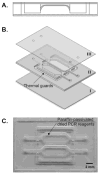
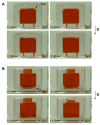
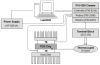
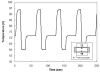
A disposable, self-contained polymerase chain reaction (PCR) chip with on-board stored, just-on-time releasable, paraffin-passivated, dry reagents is described. During both storage and sample preparation, the paraffin immobilizes and protects the stored reagents. Fluid flow through the reactor leaves the reagents undisturbed. Prior to the amplification step, the chamber is filled with target analyte suspended in water. Upon heating the PCR chamber to the DNA's denaturation temperature, the paraffin melts and moves out of the way, and the reagents are released and hydrated. To better understand the reagent release process, a scaled up model of the reactor was constructed and the paraffin migration was visualized. Experiments were carried out with a 30 microl reactor demonstrating detectable amplification (with agarose gel electrophoresis) of 10 fg ( approximately 200 copies) of lambda DNA template. The in-reactor storage and on-time release of the PCR reagents reduce the number of needed operations and significantly simplifies the flow control that would, otherwise, be needed in lab-on-chip devices.
Figures

Similar articles
-
An integrated, self-contained microfluidic cassette for isolation, amplification, and detection of nucleic acids.Biomed Microdevices. 2010 Aug;12(4):705-19. doi: 10.1007/s10544-010-9423-4. Biomed Microdevices. 2010. PMID: 20401537 Free PMC article.
-
A Multifunctional Reactor with Dry-Stored Reagents for Enzymatic Amplification of Nucleic Acids.Anal Chem. 2018 Jan 16;90(2):1209-1216. doi: 10.1021/acs.analchem.7b03834. Epub 2017 Dec 22. Anal Chem. 2018. PMID: 29226671 Free PMC article.
-
Integration of nanoparticle cell lysis and microchip PCR for one-step rapid detection of bacteria.Biomed Microdevices. 2012 Apr;14(2):337-46. doi: 10.1007/s10544-011-9610-y. Biomed Microdevices. 2012. PMID: 22094824
-
PCR microfluidic devices for DNA amplification.Biotechnol Adv. 2006 May-Jun;24(3):243-84. doi: 10.1016/j.biotechadv.2005.10.002. Epub 2005 Dec 2. Biotechnol Adv. 2006. PMID: 16326063 Review.
-
High-throughput on-chip leukemia diagnosis.Int J Lab Hematol. 2013 Oct;35(5):480-90. doi: 10.1111/ijlh.12054. Epub 2013 Feb 18. Int J Lab Hematol. 2013. PMID: 23414350 Review.
Cited by
-
Sample-to-Answer Droplet Magnetofluidic Platform for Point-of-Care Hepatitis C Viral Load Quantitation.Sci Rep. 2018 Jun 28;8(1):9793. doi: 10.1038/s41598-018-28124-3. Sci Rep. 2018. PMID: 29955160 Free PMC article.
-
An integrated, self-contained microfluidic cassette for isolation, amplification, and detection of nucleic acids.Biomed Microdevices. 2010 Aug;12(4):705-19. doi: 10.1007/s10544-010-9423-4. Biomed Microdevices. 2010. PMID: 20401537 Free PMC article.
-
A disposable, integrated loop-mediated isothermal amplification cassette with thermally actuated valves.Microfluid Nanofluidics. 2011;11(2):209-220. doi: 10.1007/s10404-011-0788-3. Epub 2011 Mar 18. Microfluid Nanofluidics. 2011. PMID: 32214952 Free PMC article.
-
Microfluidic devices for nucleic acid (NA) isolation, isothermal NA amplification, and real-time detection.Methods Mol Biol. 2015;1256:15-40. doi: 10.1007/978-1-4939-2172-0_2. Methods Mol Biol. 2015. PMID: 25626529 Free PMC article.
-
A low-cost microfluidic chip for rapid genotyping of malaria-transmitting mosquitoes.PLoS One. 2012;7(8):e42222. doi: 10.1371/journal.pone.0042222. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22879919 Free PMC article.
References
-
- Holland CA, Kiechle FL. Curr Opin Microbiol. 2005;8:504–509. - PubMed
-
- Malamud D, Bau H, Niedbala S, Corstjens P. Adv Dent Res. 2005;18:12–16. - PubMed
-
- Inganäs M, Derand H, Eckersten A, Ekstrand G, Honerud AK, Jesson G, Thorsen G, Söderman T, Andersson P. Clin Chem. 2005;51:1985–1989. - PubMed
-
- Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Nature. 2006;442:412–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

