Proteomic identification of altered apolipoprotein patterns in pulmonary hypertension and vasculopathy of sickle cell disease
- PMID: 19023114
- PMCID: PMC2635077
- DOI: 10.1182/blood-2008-03-142604
Proteomic identification of altered apolipoprotein patterns in pulmonary hypertension and vasculopathy of sickle cell disease
Abstract
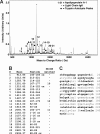
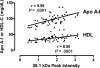
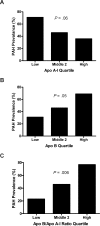
Pulmonary arterial hypertension (PAH) is emerging as a major complication and independent risk factor for death among adults with sickle cell disease (SCD). Using surface-enhanced laser desorption/ionization time of flight mass spectrometry (SELDI-TOF MS), we searched for biomarkers of PAH in plasma specimens from 27 homozygous sickle cell anemia (HbSS) patients with PAH and 28 without PAH. In PAH patients, analysis consistently showed lower abundance of a 28.1-kDa peak (P < .001), identified by high-resolution mass spectrometry as the oxidant-scavenging protein apolipoprotein A-I (apoA-I), which correlated with clinical assays of apoA-I (r = .58, P < .001) and high-density lipoprotein (HDL) levels (r = .50, P = .001). Consistent with endothelial dysfunction that may mediate this effect in PAH, HbSS patients with lower apoA-I levels also displayed impaired vasodilatory responses to acetylcholine (mean +/- SEM, 189% +/- 34% [n = 13] vs 339% +/- 51% [n = 13], P < .001). As a group, patients with SCD demonstrated significantly lower apoA-I levels than African-American control subjects. The PAH cohort was further characterized by high levels of apolipoproteins A-II and B and serum amyloid A, and low levels of haptoglobin dimers and plasminogen. These results imply a relationship of apolipoproteins to the development of PAH vasculopathy in SCD, potentially involving an unexpected mechanistic parallel to atherosclerosis, another proliferative vasculopathy.
Figures

 ).
).

 ) demonstrated dose-dependent vasodilation to ACh close to previously published normal values. In sharp contrast, those with lower than median levels of apoA-I had markedly blunted responses measured as (A) absolute blood flow (P < .001, 2-way ANOVA) or as (B) percentage of increase from baseline (P = .001). Although the (C) absolute blood flow was lower at baseline and all doses of SNP in low apoA-I patients (P = .007), the (D) percentage increase from baseline did not differ by apoA-I status.
) demonstrated dose-dependent vasodilation to ACh close to previously published normal values. In sharp contrast, those with lower than median levels of apoA-I had markedly blunted responses measured as (A) absolute blood flow (P < .001, 2-way ANOVA) or as (B) percentage of increase from baseline (P = .001). Although the (C) absolute blood flow was lower at baseline and all doses of SNP in low apoA-I patients (P = .007), the (D) percentage increase from baseline did not differ by apoA-I status.Similar articles
-
Apolipoprotein A-I and serum amyloid A plasma levels are biomarkers of acute painful episodes in patients with sickle cell disease.Haematologica. 2010 Sep;95(9):1467-72. doi: 10.3324/haematol.2009.018044. Epub 2010 Apr 7. Haematologica. 2010. PMID: 20378559 Free PMC article.
-
Lipid levels in sickle-cell disease associated with haemolytic severity, vascular dysfunction and pulmonary hypertension.Br J Haematol. 2010 May;149(3):436-45. doi: 10.1111/j.1365-2141.2010.08109.x. Epub 2010 Mar 8. Br J Haematol. 2010. PMID: 20230401 Free PMC article.
-
Altered HDL particle in sickle cell disease: decreased cholesterol content is associated with hemolysis, whereas decreased Apolipoprotein A1 is linked to inflammation.Lipids Health Dis. 2019 Dec 20;18(1):225. doi: 10.1186/s12944-019-1174-5. Lipids Health Dis. 2019. PMID: 31861992 Free PMC article.
-
Evolution of novel small-molecule therapeutics targeting sickle cell vasculopathy.JAMA. 2008 Dec 10;300(22):2638-46. doi: 10.1001/jama.2008.598. JAMA. 2008. PMID: 19066384 Free PMC article. Review.
-
Pathophysiology and treatment of pulmonary hypertension in sickle cell disease.Blood. 2016 Feb 18;127(7):820-8. doi: 10.1182/blood-2015-08-618561. Epub 2016 Jan 12. Blood. 2016. PMID: 26758918 Free PMC article. Review.
Cited by
-
Featured Article: Alterations of lecithin cholesterol acyltransferase activity and apolipoprotein A-I functionality in human sickle blood.Exp Biol Med (Maywood). 2016 Nov;241(17):1933-1942. doi: 10.1177/1535370216657447. Epub 2016 Jun 27. Exp Biol Med (Maywood). 2016. PMID: 27354333 Free PMC article.
-
Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions.Am J Hematol. 2009 Sep;84(9):618-25. doi: 10.1002/ajh.21475. Am J Hematol. 2009. PMID: 19610078 Free PMC article.
-
The proteome of sickle cell disease: insights from exploratory proteomic profiling.Expert Rev Proteomics. 2010 Dec;7(6):833-48. doi: 10.1586/epr.10.88. Expert Rev Proteomics. 2010. PMID: 21142886 Free PMC article. Review.
-
Biomarkers of World Trade Center Particulate Matter Exposure: Physiology of Distal Airway and Blood Biomarkers that Predict FEV₁ Decline.Semin Respir Crit Care Med. 2015 Jun;36(3):323-33. doi: 10.1055/s-0035-1547349. Epub 2015 May 29. Semin Respir Crit Care Med. 2015. PMID: 26024341 Free PMC article. Review.
-
Clinical biomarkers in sickle cell disease.Saudi J Biol Sci. 2015 Jan;22(1):24-31. doi: 10.1016/j.sjbs.2014.09.005. Epub 2014 Sep 18. Saudi J Biol Sci. 2015. PMID: 25561879 Free PMC article. Review.
References
-
- Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. - PubMed
-
- Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. - PubMed
-
- Ataga KI, Sood N, De GG, et al. Pulmonary hypertension in sickle cell disease. Am J Med. 2004;117:665–669. - PubMed
-
- Powars D, Weidman JA, Odom-Maryon T, Niland JC, Johnson C. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine (Baltimore) 1988;67:66–76. - PubMed
-
- Sutton LL, Castro O, Cross DJ, Spencer JE, Lewis JF. Pulmonary hypertension in sickle cell disease. Am J Cardiol. 1994;74:626–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

