Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica
- PMID: 18403674
- PMCID: PMC2396588
- DOI: 10.1126/science.1156995
Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica
Abstract
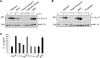
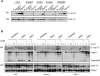
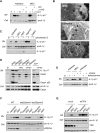
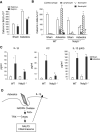
The inhalation of airborne pollutants, such as asbestos or silica, is linked to inflammation of the lung, fibrosis, and lung cancer. How the presence of pathogenic dust is recognized and how chronic inflammatory diseases are triggered are poorly understood. Here, we show that asbestos and silica are sensed by the Nalp3 inflammasome, whose subsequent activation leads to interleukin-1beta secretion. Inflammasome activation is triggered by reactive oxygen species, which are generated by a NADPH oxidase upon particle phagocytosis. (NADPH is the reduced form of nicotinamide adenine dinucleotide phosphate.) In a model of asbestos inhalation, Nalp3-/- mice showed diminished recruitment of inflammatory cells to the lungs, paralleled by lower cytokine production. Our findings implicate the Nalp3 inflammasome in particulate matter-related pulmonary diseases and support its role as a major proinflammatory "danger" receptor.
Figures
Comment in
-
Immunology. How frustration leads to inflammation.Science. 2008 May 2;320(5876):619-20. doi: 10.1126/science.1158398. Science. 2008. PMID: 18451288 No abstract available.
Similar articles
-
The Nalp3 inflammasome is essential for the development of silicosis.Proc Natl Acad Sci U S A. 2008 Jul 1;105(26):9035-40. doi: 10.1073/pnas.0803933105. Epub 2008 Jun 24. Proc Natl Acad Sci U S A. 2008. PMID: 18577586 Free PMC article.
-
Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization.Nat Immunol. 2008 Aug;9(8):847-56. doi: 10.1038/ni.1631. Epub 2008 Jul 11. Nat Immunol. 2008. PMID: 18604214 Free PMC article.
-
Orthopedic wear debris mediated inflammatory osteolysis is mediated in part by NALP3 inflammasome activation.J Orthop Res. 2013 Jan;31(1):73-80. doi: 10.1002/jor.22190. Epub 2012 Aug 29. J Orthop Res. 2013. PMID: 22933241
-
The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases.Part Fibre Toxicol. 2016 Sep 20;13(1):51. doi: 10.1186/s12989-016-0162-4. Part Fibre Toxicol. 2016. PMID: 27650313 Free PMC article. Review.
-
The inflammasome: a danger sensing complex triggering innate immunity.Curr Opin Immunol. 2007 Dec;19(6):615-22. doi: 10.1016/j.coi.2007.09.002. Epub 2007 Oct 30. Curr Opin Immunol. 2007. PMID: 17977705 Review.
Cited by
-
Monocyte- and macrophage-targeted NADPH oxidase mediates antifungal host defense and regulation of acute inflammation in mice.J Immunol. 2013 Apr 15;190(8):4175-84. doi: 10.4049/jimmunol.1202800. Epub 2013 Mar 15. J Immunol. 2013. PMID: 23509361 Free PMC article.
-
The interplay between autophagy and ROS in tumorigenesis.Front Oncol. 2012 Nov 21;2:171. doi: 10.3389/fonc.2012.00171. eCollection 2012. Front Oncol. 2012. PMID: 23181220 Free PMC article.
-
Cholesterol crystals and inflammation.Curr Rheumatol Rep. 2013 Mar;15(3):313. doi: 10.1007/s11926-012-0313-z. Curr Rheumatol Rep. 2013. PMID: 23412688 Free PMC article. Review.
-
IL-1 family cytokines trigger sterile inflammatory disease.Front Immunol. 2012 Oct 9;3:315. doi: 10.3389/fimmu.2012.00315. eCollection 2012. Front Immunol. 2012. PMID: 23087690 Free PMC article.
-
Inflammasome components Asc and caspase-1 mediate biomaterial-induced inflammation and foreign body response.Proc Natl Acad Sci U S A. 2011 Dec 13;108(50):20095-100. doi: 10.1073/pnas.1105152108. Epub 2011 Nov 22. Proc Natl Acad Sci U S A. 2011. PMID: 22109549 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

