Brain-computer interfaces: communication and restoration of movement in paralysis
- PMID: 17234696
- PMCID: PMC2151357
- DOI: 10.1113/jphysiol.2006.125633
Brain-computer interfaces: communication and restoration of movement in paralysis
Abstract
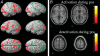
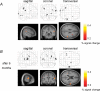
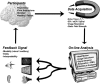
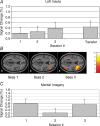
The review describes the status of brain-computer or brain-machine interface research. We focus on non-invasive brain-computer interfaces (BCIs) and their clinical utility for direct brain communication in paralysis and motor restoration in stroke. A large gap between the promises of invasive animal and human BCI preparations and the clinical reality characterizes the literature: while intact monkeys learn to execute more or less complex upper limb movements with spike patterns from motor brain regions alone without concomitant peripheral motor activity usually after extensive training, clinical applications in human diseases such as amyotrophic lateral sclerosis and paralysis from stroke or spinal cord lesions show only limited success, with the exception of verbal communication in paralysed and locked-in patients. BCIs based on electroencephalographic potentials or oscillations are ready to undergo large clinical studies and commercial production as an adjunct or a major assisted communication device for paralysed and locked-in patients. However, attempts to train completely locked-in patients with BCI communication after entering the complete locked-in state with no remaining eye movement failed. We propose that a lack of contingencies between goal directed thoughts and intentions may be at the heart of this problem. Experiments with chronically curarized rats support our hypothesis; operant conditioning and voluntary control of autonomic physiological functions turned out to be impossible in this preparation. In addition to assisted communication, BCIs consisting of operant learning of EEG slow cortical potentials and sensorimotor rhythm were demonstrated to be successful in drug resistant focal epilepsy and attention deficit disorder. First studies of non-invasive BCIs using sensorimotor rhythm of the EEG and MEG in restoration of paralysed hand movements in chronic stroke and single cases of high spinal cord lesions show some promise, but need extensive evaluation in well-controlled experiments. Invasive BMIs based on neuronal spike patterns, local field potentials or electrocorticogram may constitute the strategy of choice in severe cases of stroke and spinal cord paralysis. Future directions of BCI research should include the regulation of brain metabolism and blood flow and electrical and magnetic stimulation of the human brain (invasive and non-invasive). A series of studies using BOLD response regulation with functional magnetic resonance imaging (fMRI) and near infrared spectroscopy demonstrated a tight correlation between voluntary changes in brain metabolism and behaviour.
Figures



Similar articles
-
Breaking the silence: brain-computer interfaces (BCI) for communication and motor control.Psychophysiology. 2006 Nov;43(6):517-32. doi: 10.1111/j.1469-8986.2006.00456.x. Psychophysiology. 2006. PMID: 17076808 Review.
-
Physiological regulation of thinking: brain-computer interface (BCI) research.Prog Brain Res. 2006;159:369-91. doi: 10.1016/S0079-6123(06)59024-7. Prog Brain Res. 2006. PMID: 17071243 Review.
-
Brain-computer interface in paralysis.Curr Opin Neurol. 2008 Dec;21(6):634-8. doi: 10.1097/WCO.0b013e328315ee2d. Curr Opin Neurol. 2008. PMID: 18989104 Review.
-
Brain-computer interfaces in the completely locked-in state and chronic stroke.Prog Brain Res. 2016;228:131-61. doi: 10.1016/bs.pbr.2016.04.019. Epub 2016 Aug 8. Prog Brain Res. 2016. PMID: 27590968 Review.
-
Brain-machine interface (BMI) in paralysis.Ann Phys Rehabil Med. 2015 Feb;58(1):9-13. doi: 10.1016/j.rehab.2014.11.002. Epub 2015 Jan 8. Ann Phys Rehabil Med. 2015. PMID: 25623294
Cited by
-
Combining Real-Time fMRI Neurofeedback Training of the DLPFC with N-Back Practice Results in Neuroplastic Effects Confined to the Neurofeedback Target Region.Front Behav Neurosci. 2016 Jun 28;10:138. doi: 10.3389/fnbeh.2016.00138. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27445733 Free PMC article.
-
Self-Regulation of Blood Oxygenation Level Dependent Response: Primary Effect or Epiphenomenon?Front Neurosci. 2016 Mar 24;10:117. doi: 10.3389/fnins.2016.00117. eCollection 2016. Front Neurosci. 2016. PMID: 27047332 Free PMC article. No abstract available.
-
Neural Signature and Decoding of Unmanned Aerial Vehicle Operators in Emergency Scenarios Using Electroencephalography.Sensors (Basel). 2024 Sep 29;24(19):6304. doi: 10.3390/s24196304. Sensors (Basel). 2024. PMID: 39409342 Free PMC article.
-
Progress and prospects in neurorehabilitation: clinical applications of stem cells and brain-computer interface for spinal cord lesions.Neurol Sci. 2013 Apr;34(4):427-33. doi: 10.1007/s10072-012-1232-5. Epub 2012 Nov 17. Neurol Sci. 2013. PMID: 23161257 Review.
-
Protease-degradable PEG-maleimide coating with on-demand release of IL-1Ra to improve tissue response to neural electrodes.Biomaterials. 2015 Mar;44:55-70. doi: 10.1016/j.biomaterials.2014.12.009. Epub 2015 Jan 12. Biomaterials. 2015. PMID: 25617126 Free PMC article.
References
-
- Adam G. Visceral Perception. New York: Plenum Press; 1998.
-
- Bandura A. Social learning of moral judgements. J Pers Soc Psychol. 1969;11:275–279. - PubMed
-
- Barber TX, Kamiya J, Miller NE. Biofeedback and Self-Control. Chicago: Aldine Series, Aldine; 1971–78.
-
- Berger H. Ueber das Elektrenkephalogramm des Menschen. Arch Psychiatrie Nervenkrankheiten. 1929;87:527–570.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

