Key Points
-
The cerebellum is traditionally regarded as a structure involved in motor control, but it is becoming increasingly clear that it also has an important role in processing higher level 'cognitive' information.
-
This review first summarizes the anatomy of the cortico-cerebellar system, arguing that important clues about information processing can be derived from knowledge of its structural organization.
-
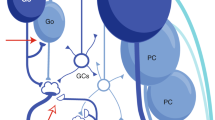
The microstructure of the cerebellar cortex is uniform, suggesting that it processes its diverse inputs using a common set of computational principles.
-
Control theory provides an excellent way to explain the involvement of the cerebellum in the control of movement.
-
The anatomical organization of the cortico-cerebellar system suggests that these control theoretic accounts can be extended to explain how cerebellar circuits process information from the prefrontal cortex
Abstract
Evidence has been accumulating that the primate cerebellum contributes not only to motor control, but also to higher 'cognitive' function. However, there is no consensus about how the cerebellum processes such information. The answer to this puzzle can be found in the nature of cerebellar connections to areas of the cerebral cortex (particularly the prefrontal cortex) and in the uniformity of its intrinsic cellular organization, which implies uniformity in information processing regardless of the area of origin in the cerebral cortex. With this in mind, the relatively well-developed models of how the cerebellum processes information from the motor cortex might be extended to explain how it could also process information from the prefrontal cortex.
This is a preview of subscription content, access via your institution
Access options
Subscription info for Japanese customers
We have a dedicated website for our Japanese customers. Please go to natureasia.com to subscribe to this journal.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Stein, J. F. & Glickstein, M. Role of the cerebellum in visual guidance of movement. Physiol. Rev. 72, 967–1017 (1992).
Brodal, P. & Bjaalie, J. G. Salient anatomic features of the cortico-ponto-cerebellar pathway. Prog. Brain Res. 114, 227–249 (1997).
Glickstein, M. Motor skills but not cognitive tasks. Trends Neurosci. 16, 450–451 (1993).
Glickstein, M. The cerebellum and motor learning. Curr. Opin. Neurobiol. 2, 802–806 (1992).
Fine, E. J., Ionita, C. C. & Lohr, L. The history of the development of the cerebellar examination. Semin. Neurol. 22, 375–384 (2002).
Holmes, G. The cerebellum of man. Brain 62, 1–30 (1939).
Ito, M. Historical review of the significance of the cerebellum and the role of Purkinje cells in motor learning. Ann. NY Acad. Sci. 978, 273–288 (2002).
Fiez, J. A. et al. A positron emission tomography study of the short-term maintenance of verbal information. J. Neurosci. 16, 808–822 (1996).
Allen, G., Buxton, R. B., Wong, E. C. & Courchesne, E. Attentional activation of the cerebellum independent of motor involvement. Science 275, 1940–1943 (1997).
Ivry, R. B. & Baldo, J. V. Is the cerebellum involved in learning and cognition? Curr. Opin. Neurobiol. 2, 212–216 (1992).
Kim, S. G., Ugurbil, K. & Strick, P. L. Activation of a cerebellar output nucleus during cognitive processing. Science 265, 949–951 (1994).
Schmahmann, J. D. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J. Neuropsychiatry Clin. Neurosci. 16, 367–378 (2004). Discusses clinical evidence that cerebellar lesions in the human brain produce deficits in cognitive function.
Desmond, J. E., Gabrieli, J. D. & Glover, G. H. Dissociation of frontal and cerebellar activity in a cognitive task: evidence for a distinction between selection and search. Neuroimage 7, 368–376 (1998).
Chen, S. H. & Desmond, J. E. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage 24, 332–338 (2005).
Ito, M. Mechanisms of motor learning in the cerebellum. Brain Res. 886, 237–245 (2000).
Albus, J. S. A theory of cerebellar function. Math. Biosci. 10, 25–61 (1971).
Blomfield, S. & Marr, D. How the cerebellum may be used. Nature 227, 1224–1228 (1970).
Marr, D. A theory of cerebellar cortex. J. Physiol. 202, 437–470 (1969). Contains some of the most influential theoretical ideas related to cerebellar information processing.
Eccles, J. C., Ito, M. & Szentagothai, J. The Cerebellum as a Neuronal Machine (Springer, New York, 1967).
Bloedel, J. R. Functional heterogeneity with structural homogeneity: how does the cerebellum operate? Behav. Brain Sci. 15, 666–678 (1992).
Zagon, I. S., McLaughlin, P. J. & Smith, S. Neural populations in the human cerebellum: estimations from isolated cell nuclei. Brain Res. 127, 279–282 (1977).
Apps, R. & Garwicz, M. Anatomical and physiological foundations of cerebellar information processing. Nature Rev. Neurosci. 6, 297–311 (2005).
Larsell, O. & Jansen, O. The Comparative Anatomy and Histology of the Cerebellum: the Human Cerebellum, Cerebellar Connections and Cerebellar Cortex (University of Minnesota Press, Minneapolis, 1972).
Larsell, O. Comparative Anatomy and Histology of the Cerebellum from Monotremes through Apes (University of Minnesota Press, Minneapolis, 1970).
Fox, C. A. & Barnard, J. W. A quantitative study of the Purkinje cell dendritic branchlets and their relationship to afferent fibres. J. Anat. 91, 299–313 (1957).
Fuster, J. M. Upper processing stages of the perception-action cycle. Trends Cogn. Sci. 8, 143–145 (2004).
Shah, V. S., Schmahmann, J. D., Pandya, D. N. & Vaher, P. R. Associative projections to the Zona Incerta: possible anatomic substrate for extension of the Marr–Albus hypothesis to non-motor learning. Soc. Neurosci. 23, 1829 (1997).
Middleton, F. A. & Strick, P. L. Cerebellar output channels. Int. Rev. Neurobiol. 4161–4182 (1997).
Thach, W. T. Cerebellar output: properties, synthesis and uses. Brain Res. 40, 89–102 (1972).
Thach, W. T. & Jones, E. G. The cerebellar dentatothalamic connection: terminal field, lamellae, rods and somatotopy. Brain Res. 169, 168–172 (1979).
Asanuma, C., Thach, W. T. & Jones, E. G. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res. 286, 237–265 (1983).
Brodal, P. Principles of organization of the monkey corticopontine projection. Brain Res. 148, 214–218 (1978).
Schmahmann, J. D. & Pandya, D. N. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J. Neurosci. 17, 438–458 (1997). The authors have characterized patterns of projections from several prefrontal areas to the pontine nuclei.
Schmahmann, J. D., Rosene, D. L. & Pandya, D. N. Motor projections to the basis pontis in rhesus monkey. J. Comp. Neurol. 478, 248–268 (2004).
Glickstein, M., May, J. G. & Mercier, B. E. Corticopontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J. Comp. Neurol. 235, 343–359 (1985).
Brodal, P. The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain 101, 251–283 (1978).
Walker, A. E. A cytoarchitectural study of the prefrontal area of the macaque monkey. J. Comp. Neurol. 73, 59–86 (1940).
Kelly, R. M. & Strick, P. L. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 23, 8432–8444 (2003). The only study in the literature so far that has identified the trans-synaptic pathways from motor and prefrontal areas to distinct locations in the cerebellar cortex in the primate brain. The importance of this study lies in the fact that it allows researchers to generate anatomically specific hypotheses about the operations of specific modules in the cerebellar cortex.
Middleton, F. A. & Strick, P. L. Cerebellar projections to the prefrontal cortex of the primate. J. Neurosci. 21, 700–712 (2001).
Petrides, M. & Pandya, D. N. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur. J. Neurosci. 11, 1011–1036 (1999).
Petrides, M. & Pandya, D. N. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 16, 291–310 (2002).
Fuster, J. M. Linkage at the top. Neuron 21, 1223–1224 (1998).
Orioli, P. J. & Strick, P. L. Cerebellar connections with the motor cortex and the arcuate premotor area: an analysis employing retrograde transneuronal transport of WGA-HRP. J. Comp. Neurol. 288, 612–626 (1989).
Lu, M. T., Preston, J. B. & Strick, P. L. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J. Comp. Neurol. 341, 375–392 (1994).
Passingham, R. E. Attention to action. Phil. Trans. R. Soc. Lond. B Biol. Sci. 351, 1473–1479 (1996).
Rosa, M. G. & Tweedale, R. Brain maps, great and small: lessons from comparative studies of primate visual cortical organization. Phil. Trans. R. Soc. Lond. B Biol. Sci. 360, 665–691 (2005).
Barton, R. A. & Harvey, P. H. Mosaic evolution of brain structure in mammals. Nature 405, 1055–1058 (2000).
Schoenemann, P. T., Sheehan, M. J. & Glotzer, L. D. Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nature Neurosci. 8, 242–252 (2005).
Matano, S. Brief communication: proportions of the ventral half of the cerebellar dentate nucleus in humans and great apes. Am. J. Phys. Anthropol. 114, 163–165 (2001).
Beck, E. The origin, course and termination of the prefrontopontine tract in the human brain. Brain 7, 368–391 (1950).
Dejerine, J. Anatomie des Centres Nerveux (Rueff et Cie, Paris, 1895).
Marin, O. S. M., Angevine, J. B. & Locke, S. Topographical organisation of the lateral segment of the basis pedunculi in man. J. Comp. Neurol. 118, 165–175 (1962).
Behrens, T. E. et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 50, 1077–1088 (2003).
Le Bihan, D. Looking into the functional architecture of the brain with diffusion MRI. Nature Rev. Neurosci. 4, 469–480 (2003).
Ramnani, N., Behrens, T. E., Penny, W. & Matthews, P. M. New approaches for exploring anatomical and functional connectivity in the human brain. Biol. Psychiatry 56, 613–619 (2004).
Behrens, T. E. et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neurosci. 6, 750–757 (2003).
Ramnani, N. et al. The evolution of prefrontal inputs to the cortico-pontine system: diffusion imaging evidence from macaque monkeys and humans. Cereb. Cortex 16, 811–818 (2006). The same anatomical method was used to investigate cortico-pontine projections from various cortical areas in both humans and macaque monkeys. This study demonstrated a selective enlargement of prefrontal contributions to this system in the human brain.
Kawato, M. & Wolpert, D. Internal models for motor control. Novartis Found. Symp. 218, 291–304, 304–307 (1998).
Brindely, G. S. The use made by the cerebellum of the information that it receives from the sense organs. Int. Brain Res. Org. Bull. 3, 80 (1969).
Boyden, E. S., Katoh, A. & Raymond, J. L. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu. Rev. Neurosci. 27, 581–609 (2004).
Ito, M. Synaptic plasticity in the cerebellar cortex and its role in motor learning. Can. J. Neurol. Sci. 20, S70–S74 (1993).
Ito, M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol. Rev. 81, 1143–1195 (2001).
De Zeeuw, C. I. & Yeo, C. H. Time and tide in cerebellar memory formation. Curr. Opin. Neurobiol. 15, 667–674 (2005).
Horn, K. M., Pong, M. & Gibson, A. R. Discharge of inferior olive cells during reaching errors and perturbations. Brain Res. 996, 148–158 (2004).
Garwicz, M. Spinal reflexes provide motor error signals to cerebellar modules — relevance for motor coordination. Brain Res. Brain Res. Rev. 40, 152–165 (2002).
Krupa, D. J., Thompson, J. K. & Thompson, R. F. Localization of a memory trace in the mammalian brain. Science 260, 989–991 (1993).
Ebner, T. J., Johnson, M. T., Roitman, A. & Fu, Q. What do complex spikes signal about limb movements? Ann. NY Acad. Sci. 978, 205–218 (2002).
De Zeeuw, C. I. et al. Microcircuitry and function of the inferior olive. Trends Neurosci. 21, 391–400 (1998).
Miall, R. C., Weir, D. J., Wolpert, D. M. & Stein, J. F. Is the cerebellum a smith predictor? J. Mot. Behav. 25, 203–216 (1993).
Ohyama, T., Nores, W. L., Murphy, M. & Mauk, M. D. What the cerebellum computes. Trends Neurosci. 26, 222–227 (2003).
Braitenberg, V., Heck, D. & Sultan, F. The detection and generation of sequences as a key to cerebellar function: experiments and theory. Behav. Brain Sci. 20, 229–277 (1997).
Thach, W. T. Context-response linkage. Int. Rev. Neurobiol. 41, 599–611 (1997).
Thach, W. T., Goodkin, H. P. & Keating, J. G. The cerebellum and the adaptive coordination of movement. Annu. Rev. Neurosci. 15, 403–442 (1992).
Ito, M. Movement and thought: identical control mechanisms by the cerebellum. Trends Neurosci. 16, 448–450; 453–454 (1993).
Ito, M. Bases and implications of learning in the cerebellum — adaptive control and internal model mechanism. Prog. Brain Res. 148, 95–109 (2005). Highlights some recent control theoretic ideas on how prefrontal interactions with posterior association areas are modelled by prefrontal interactions with cerebellar cortical circuits.
Barlow, J. S. The Cerebellum and Adaptive Control (Cambridge Univ. Press, Cambridge, UK, 2002).
Wolpert, D. M. & Miall, R. C. Forward models for physiological motor control. Neural Netw. 9, 1265–1279 (1996). A clear account of forward models, and of how these might be modelled in cerebellar circuitry.
Allen, G. I. & Tsukahara, N. Cerebrocerebellar communication systems. Physiol. Rev. 54, 957–1006 (1974).
Wolpert, D. M. & Kawato, M. Multiple paired forward and inverse models for motor control. Neural Netw. 11, 1317–1329 (1998).
Jordan, M. I. in The Cognitive Neurosciences (ed. Gazzaniga, M.) 579–609 (MIT Press, Cambridge, Massachusetts, 1995).
Jordan, M. I. & Rumelhart, D. E. Forward models: supervised learning wih a distal teacher. Cogn Sci. 16, 307–354 (1992).
Kawato, M., Furukawa, K. & Suzuki, R. A hierarchical neural-network model for control and learning of voluntary movement. Biol. Cybern. 57, 169–185 (1987).
Linden, D. J. Neuroscience. From molecules to memory in the cerebellum. Science 301, 1682–1685 (2003).
Georgopoulos, A. P. Higher order motor control. Annu. Rev. Neurosci. 14, 361–377 (1991).
He, S. Q., Dum, R. P. & Strick P. L. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J. Neurosci. 13, 952–980 (1993).
He, S. Q., Dum, R. P. & Strick, P. L. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. J. Neurosci. 15, 3284–3306 (1995).
Dum, R. P. & Strick, P. L. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J. Neurosci. 16, 6513–6525 (1996).
Ugolini, G. & Kuypers, H. G. Collaterals of corticospinal and pyramidal fibres to the pontine grey demonstrated by a new application of the fluorescent fibre labelling technique. Brain Res. 365, 211–227 (1986).
Thach, W. T. Cerebellar inputs to motor cortex. Ciba Found. Symp. 132, 201–220 (1987).
Dum, R. P., Li, C. & Strick, P. L. Motor and nonmotor domains in the monkey dentate. Ann. NY Acad. Sci. 978, 289–301 (2002).
Dum, R. P. & Strick, P. L. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J. Neurophysiol. 89, 634–639 (2003).
Cheney, P. D., Fetz, E. E. & Mewes, K. Neural mechanisms underlying corticospinal and rubrospinal control of limb movements. Prog. Brain Res. 87, 213–252 (1991).
Nathan, P. W. & Smith, M. C. The rubrospinal and central tegmental tracts in man. Brain 105, 223–269 (1982).
Ralston, D. D. Cerebellar terminations in the red nucleus of Macaca fascicularis: an electron-microscopic study utilizing the anterograde transport of WGA:HRP. Somatosens. Mot. Res. 11, 101–107 (1994).
Buisseret-Delmas, C. An HRP study of the afferents to the inferior olive in cat. I. — Cervical spinal and dorsal column nuclei projections. Arch. Ital. Biol. 118, 270–286 (1980).
Armstrong, D. M. & Schild, R. F. Spino-olivary neurones in the lumbo-sacral cord of the cat demonstrated by retrograde transport of horseradish peroxidase. Brain Res. 168, 176–179 (1979).
Oscarsson, O. & Sjolund, B. The ventral spino-olivocerebellar system in the cat. III. Functional characteristics of the five paths. Exp. Brain Res. 28, 505–520 (1977).
Di Biagio, F. & Grundfest, H. Afferent relations of inferior olivary nucleus. II. Site of relay from hand limb afferents into dorsal spino-olivary tract in cat. J. Neurophysiol. 18, 299–304 (1955).
Ruigrok, T. J. Cerebellar nuclei: the olivary connection. Prog. Brain Res. 114, 167–192 (1997).
Courville, J. & Otabe, S. The rubro-olivary projection in the macaque: an experimental study with silver impregnation methods. J. Comp. Neurol. 158, 479–494 (1974).
Gilbert, P. F. & Thach, W. T. Purkinje cell activity during motor learning. Brain Res. 128, 309–328 (1977).
Frens, M. A., Mathoera, A. L. & van der Steen, J. Floccular complex spike response to transparent retinal slip. Neuron 30, 795–801 (2001).
Kitazawa, S., Kimura, T. & Yin, P. B. Cerebellar complex spikes encode both destinations and errors in arm movements. Nature 392, 494–497 (1998).
Bastian, A. J., Martin, T. A., Keating, J. G. & Thach, W. T. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J. Neurophysiol. 76, 492–509 (1996).
Bastian, A. J. & Thach, W. T. Cerebellar outflow lesions: a comparison of movement deficits resulting from lesions at the levels of the cerebellum and thalamus. Ann. Neurol. 38, 881–892 (1995).
Goodkin, H. P., Keating, J. G., Martin, T. A. & Thach, W. T. Preserved simple and impaired compound movement after infarction in the territory of the superior cerebellar artery. Can. J. Neurol. Sci. 20, S93–S104 (1993).
Hardiman, M. J., Ramnani, N. & Yeo, C. H. Reversible inactivations of the cerebellum with muscimol prevent the acquisition and extinction of conditioned nictitating membrane responses in the rabbit. Exp. Brain Res. 110, 235–247 (1996).
Ramnani, N. & Yeo, C. H. Reversible inactivations of the cerebellum prevent the extinction of conditioned nictitating membrane responses in rabbits. J. Physiol. 495, 159–168 (1996).
Lu, X., Hikosaka, O. & Miyachi, S. Role of monkey cerebellar nuclei in skill for sequential movement. J. Neurophysiol. 79, 2245–2254 (1998).
Meyer, J. S., Obara, K. & Muramatsu, K. Diaschisis. Neurol. Res. 15, 362–366 (1993).
Ramnani, N., Toni, I., Josephs, O., Ashburner, J. & Passingham, R. E. Learning- and expectation-related changes in the human brain during motor learning. J. Neurophysiol. 84, 3026–3035 (2000).
Imamizu, H. et al. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403, 192–195 (2000).
Diedrichsen, J., Hashambhoy, Y., Rane, T. & Shadmehr, R. Neural correlates of reach errors. J. Neurosci. 25, 9919–9931 (2005).
Kawato, M. et al. Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Prog. Brain Res. 142, 171–188 (2003).
Kawato, M. Internal models for motor control and trajectory planning. Curr. Opin. Neurobiol. 9, 718–727 (1999).
Miall, R. C., Weir, D. J. & Stein, J. F. Manual tracking of visual targets by trained monkeys. Behav. Brain Res. 20, 185–201 (1986).
Grafton, S. T. Woods, R. P. & Tyszka, M. Functional imaging of procedural motor learning: relating cerebral blood flow with individual subject performance. Hum. Brain Mapp. 1, 221–234 (1993).
Miall, R. C. & Jenkinson, E. W. Functional imaging of changes in cerebellar activity related to learning during a novel eye-hand tracking task. Exp. Brain Res. 166, 170–183 (2005).
Ramnani, N., Toni, I., Passingham, R. E. & Haggard, P. The cerebellum and parietal cortex play a specific role in coordination: a PET study. Neuroimage 14, 899–911 (2001).
Ramnani, N. & Passingham, R. E. Changes in the human brain during rhythm learning. J. Cogn. Neurosci. 13, 952–966 (2001).
Friston, K. J., Frith, C. D., Passingham, R. E., Liddle, P. F. & Frackowiak, R. S. Motor practice and neurophysiological adaptation in the cerebellum: a positron tomography study. Proc. R. Soc. Lond. B Biol. Sci. 248, 223–228 (1992).
Jueptner, M., Frith, C. D., Brooks, D. J., Frackowiak, R. S. & Passingham, R. E. Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J. Neurophysiol. 77, 1325–1337 (1997).
Passingham, R. E., Stephan, K. E. & Kotter, R. The anatomical basis of functional localization in the cortex. Nature Rev. Neurosci. 3, 606–616 (2002).
Leiner, H. C., Leiner, A. L. & Dow, R. S. Cognitive and language functions of the human cerebellum. Trends Neurosci. 16, 444–447 (1993).
Leiner, H. C., Leiner, A. L. & Dow, R. S. Does the cerebellum contribute to mental skills? Behav. Neurosci. 100, 443–454 (1986). These authors were among the first to propose a role for the cerebellum in functions that are unrelated to motor control.
Schmahmann, J. D. & Pandya, D. N. The cerebrocerebellar system. Int. Rev. Neurobiol. 4131–4160 (1997).
Wallis, J. D., Anderson, K. C. & Miller, E. K. Single neurons in prefrontal cortex encode abstract rules. Nature 411, 953–956 (2001).
Miller, E. K. The prefrontal cortex and cognitive control. Nature Rev. Neurosci. 1, 59–65 (2000).
Leiner, H. C. & Leiner, A. L. How fibers subserve computing capabilities: similarities between brains and machines. Int. Rev. Neurobiol. 41, 535–553 (1997).
Morissette, J. & Bower, J. M. Contribution of somatosensory cortex to responses in the rat cerebellar granule cell layer following peripheral tactile stimulation. Exp. Brain. Res. 109, 240–250 (1996).
Lorenzo, D., Velluti, J. C., Crispino, L. & Velluti, R. Cerebellar sensory functions. Exp. Neurol. 55, 629–636 (1977).
Schmahmann, J. D. & Sherman, J. C. The cerebellar cognitive affective syndrome. Brain 121, 561–579 (1998).
Andreasen, N. C., Paradiso, S. & O'Leary, D. S. 'Cognitive dysmetria' as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull. 24, 203–218 (1998).
Greger, B., Norris, S. A. & Thach, W. T. Spike firing in the lateral cerebellar cortex correlated with movement and motor parameters irrespective of the effector limb. J. Neurophysiol. 91, 576–582 (2004).
Parsons, L. M. & Fox, P. T. Sensory and cognitive functions. Int. Rev. Neurobiol. 41, 255–271 (1997).
Fiez, J. A., Raichle, M. E., Balota, D. A., Tallal, P. & Petersen, S. E. PET activation of posterior temporal regions during auditory word presentation and verb generation. Cereb. Cortex 6, 1–10 (1996).
Ramnani, N., Elliott, R., Athwal, B. S. & Passingham, R. E. Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage 23, 777–786 (2004).
Fiez, J. A. Cerebellar contributions to cognition. Neuron 16, 13–15 (1996).
Awh, E. et al. DIssociation of storage and rehearsal in verbal working memory: evidence from positron emission tomography. Psychol. Sci. 255, 556–559 (1996).
Desmond, J. E., Gabrieli, J. D., Wagner, A. D., Ginier, B. L. & Glover, G. H. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J. Neurosci. 17, 9675–9685 (1997).
Chen, S. H. & Desmond, J. E. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia 43, 1227–1237 (2005).
Jonides, J. et al. Verbal working memory load affects regional brain activation as measured by PET. J. Cogn. Neurosci. 9, 462–475 (1997).
Grasby, P. M. et al. Functional mapping of brain areas implicated in auditory–verbal memory function. Brain 116, 1–20 (1993).
Paulesu, E., Frith, C. D. & Frackowiak, R. S. The neural correlates of the verbal component of working memory. Nature 362, 342–345 (1993).
Paulesu, E. et al. Functional MR imaging correlations with positron emission tomography. Initial experience using a cognitive activation paradigm on verbal working memory. Neuroimaging Clin. N. Am. 5, 207–225 (1995).
Ravizza, S. M. et al. Cerebellar damage produces selective deficits in verbal working memory. Brain 129, 306–320 (2006).
Justus, T., Ravizza, S. M., Fiez, J. A. & Ivry, R. B. Reduced phonological similarity effects in patients with damage to the cerebellum. Brain Lang. 95, 304–318 (2005).
Jueptner, M. et al. Anatomy of motor learning. I. Frontal cortex and attention to action. J. Neurophysiol. 77, 1313–1324 (1997).
Kandel, E. R., Schwartz, J. H., Jessell, T. M. Principles of Neuroscience (Elsevier Science, 1991).
Dudai, Y. The Neurobiology of Memory (Oxford Univ. Press, Oxford, 1989).
Schmahmann, J. D. & Pandya, D. N. Prefrontal cortex projections to the basilar pons in rhesus monkey: implications for the cerebellar contribution to higher function. Neurosci. Lett. 199, 175–178 (1995).
Duvernoy, H. M. & Bourgouin, P. The Human Brain: Surface, Three-Dimensional Sectional Anatomy and MRI (Springer, Wein, 1999).
Gray, H. Gray's Anatomy 16th edn (Elsevier, 1994).
Acknowledgements
I would like to thank R. C. Miall and P. L. Strick for helpful discussions.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Climbing fibres
-
Axons of inferior olive neurons that extend into the cerebellar cortex and exert a powerful influence on Purkinje cells. One of two main inputs into the cerebellum.
- Crus II
-
This is the area of the macaque monkey cerebellar cortex that is most heavily interconnected with the area 46 of the prefrontal cortex.
- Cerebral peduncle
-
All cortical projections that send fibres to the pontine nuclei converge into this white matter fibre bundle before synapsing with pontine neurons. This is a convenient location at which to study the organization of cortico-pontine projections using diffusion tensor imaging.
- Efference copy
-
Information processing might require that information exchanged between two systems is monitored by a third system (as in the case of systems that incorporate control theoretic internal models). Therefore, whenever such information is exchanged, an exact copy (an efference copy) is additionally transmitted to the monitor.
- Diaschesis
-
A condition in which lesions not only impair information processing at the site of the lesion, but also adversely affect the information processing in connected downstream pathways. Therefore, the behavioural effects of lesions might at least in part be due to the impaired physiology of such areas rather than the direct effects of the lesion.
Rights and permissions
About this article
Cite this article
Ramnani, N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci 7, 511–522 (2006). https://doi.org/10.1038/nrn1953
Issue Date:
DOI: https://doi.org/10.1038/nrn1953